Nuclear RNA Export Pathways (original) (raw)
One of the most profound differences between prokaryotes and eukaryotes is that only the latter segregate RNA synthesis and protein synthesis into distinct cellular compartments, i.e., into the nucleus and cytoplasm, respectively. This segregation offers opportunities for the regulation of both gene expression and RNA posttranscriptional processing that are not available to prokaryotes. However, it also means that eukaryotic cells must continuously transport large numbers of macromolecules between the nucleus and the cytoplasm in order that these can fulfill their appointed role in the life of the cell. In this review, I will describe the mechanisms used by the cell to move different classes of cellular RNA from their nuclear site of synthesis to the cytoplasm, where the majority then participate in the process of protein synthesis. While my primary focus will be on the insights that have been gleaned from the study of nuclear RNA export in vertebrate cells, I will also refer to studies with the yeast Saccharomyces cerevisiae which, because of its genetic malleability, has proven to be a valuable system for the identification of critical components of the nucleocytoplasmic transport machinery. More comprehensive overviews of nucleocytoplasmic transport, particularly of protein import, are provided by several excellent recent reviews in this area (21, 42, 50, 70).
BASIC PRINCIPLES OF NUCLEOCYTOPLASMIC TRANSPORT
With the exception of the short period of nuclear envelope breakdown that accompanies mitosis in higher eukaryotes, all macromolecular transport between the nucleus and cytoplasm must occur via nuclear pore complexes (NPCs) (21, 42, 50, 69). While the average nucleus present in cultured human cells appears to contain ∼4,000 NPCs, this number is strongly influenced by cell size and by the level of biosynthetic and proliferative activity. The NPCs, which display a clear eightfold rotational symmetry in electron micrographs, are large, ∼125-MDa structures that protrude into both the nuclear and cytoplasmic compartments. NPCs are composed of approximately 50 to 80 distinct proteins in higher eukaryotes. A subset of these NPC components, which are termed nucleoporins, contain characteristic domains featuring multiple repeats of short peptide sequences ending in the amino acids phenylalanine and glycine (FG). These FG repeats are believed to function as transient docking sites for nucleocytoplasmic transport factors. The NPCs contain an aqueous channel that is ∼9 nm in diameter when at rest but can expand to up to ∼25 nm during active transport. The fact that the NPC remains partially open when not engaged in active transport means that small (≤40-kDa) proteins and RNAs can move between the nucleus and cytoplasm by passive diffusion. This process is, however, inefficient and even small proteins and RNAs that move between these cellular compartments are generally actively transported (11, 76).
The bulk of cellular nucleocytoplasmic transport is mediated by factors that belong to a single family of nuclear transport receptors, the prototype of which is the nuclear import factor termed importin β (Impβ) or karyopherin β1. Other prominent members of this extensive protein family include the import factor transportin (also termed karyopherin β2) and the export factors Crm1 (also termed exportin) and exportin t (Exp-t). Different members of this protein family are able to bind to distinct cargo molecules, or to adapter proteins that in turn bind cargo, and also share the ability to interact with specific nucleoporins. However, the most important characteristic of these nuclear transport receptors is their shared dependence on the biological activity of a key cofactor, the cellular G protein Ran (22, 27, 43, 46).
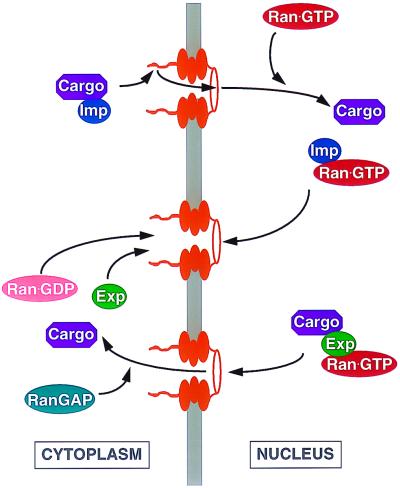
The Ran-specific GTPase-activating protein (RanGAP) is localized to the cell cytoplasm (5), while the Ran-specific guanine nucleotide exchange factor (RanGEF) is localized to the nucleus (6). As a result, cytoplasmic Ran is predicted to exist predominantly in the GDP-bound form while nuclear Ran is largely bound to GTP. Nuclear import factors such as transportin or Impβ bind their protein cargo in the cytoplasm, in the absence of Ran-GTP, and then dock at the NPC by directly binding to one or more nucleoporins (Fig. 1). After translocation through the NPC, a process which remains poorly understood, the carrier-cargo complex reaches the inner face of the nuclear membrane and there encounters the nuclear pool of Ran-GTP. Binding of Ran-GTP to the import factor induces a conformational shift that results in the release of the cargo from the carrier and also facilitates release of the carrier from the NPC (22, 27, 59) (Fig. 1). The Ran-GTP-bound form of the carrier is then returned to the cytoplasm, via the NPC, where the Ran-GTP is hydrolyzed to Ran-GDP by the action of the RanGAP. This induces the release of the carrier protein, which is then available for further cycles of cargo import.
FIG. 1.
Nucleocytoplasmic transport by members of the Impβ protein family. Illustrated is the key role played by the Ran-GTPase cycle in both the nuclear import (upper panel) and the nuclear export (lower panel) of many cargo proteins and RNAs. See the text for a detailed discussion. Imp, import factor; Exp, export factor.
Nuclear export by members of the Impβ family of nuclear transport receptors, such as Crm1, is also highly dependent upon the Ran-GTP cofactor. However, in this case, Ran-GTP is essential for the initial interaction of the export factor with its cargo in the nucleus (18, 34) (Fig. 1). Export again is mediated by docking of the export factor at the NPC, followed by translocation. Release of the cargo occurs upon hydrolysis of the Ran-GTP to Ran-GDP by cytoplasmic RanGAP. Both the export carrier and the Ran-GDP are then recycled back to the nucleus, where the latter is converted to Ran-GTP by RanGEF (Fig. 1). Because the majority of nuclear import and export is mediated by Impβ family members, interference with the Ran-GTP gradient across the nuclear membrane affects many transport processes. This inhibition may be either direct or indirect, for example, by inhibiting the nuclear import of a factor required for the nuclear export of a particular substrate, and the mechanistic interpretation of such an inhibition can therefore be difficult.
Several different types of RNA are exported from the nucleus to the cytoplasm, including transcripts synthesized by RNA polymerase I (large rRNAs), RNA polymerase II (mRNAs and some uridine-rich small nuclear RNAs [U snRNAs]), and RNA polymerase III (tRNA and 5S rRNA). By competition experiments with microinjected Xenopus oocytes, it was shown that these RNAs largely use distinct pathways to exit the nucleus; e.g., an excess of microinjected tRNA inhibits tRNA export but not U snRNA or mRNA export (29). Efforts to identify the critical protein components that define each of these distinct nuclear RNA export pathways have utilized a number of distinct yet complementary approaches, including genetic analysis of yeast cells and biochemical analysis using vertebrate cell extracts. A system that has proven to be particularly useful in this regard is the analysis of retroviral nuclear RNA export pathways.
NUCLEAR EXPORT OF HIV-1 RNAS
The large majority of cellular mRNAs are expressed as pre-mRNAs containing intronic regions that must be removed by splicing prior to export and translation. Because the inappropriate nuclear export of an incompletely spliced mRNA would likely result in the synthesis of a protein product that would be nonfunctional or even actively deleterious, cells have stringent quality control mechanisms to ensure that only fully spliced mRNAs exit the nucleus. This primarily reflects the recognition of splice sites by a subset of splicing factors, termed commitment factors, that actively retain incompletely spliced mRNAs in the nucleus (12, 35).
While clearly advantageous to the cell, this presents a problem to retroviruses that, as an integral part of their life cycle, must express both fully spliced and incompletely spliced variants of the same initial transcript in the cytoplasm of the infected cell (13). Retroviruses have therefore had to evolve novel ways to promote the sequence-specific nuclear export of their incompletely spliced mRNAs in the face of this nuclear retention mechanism. Investigation of the mechanisms used by different retroviruses to achieve this goal has yielded important insights into how the cell itself regulates nuclear RNA export (13).
The first sequence-specific nuclear RNA export factor to be identified was the human immunodeficiency virus type 1 (HIV-1) Rev protein, and Rev continues to serve as a valuable paradigm. Like many other retroviruses, HIV-1 expresses only a single initial transcript which must then be exported to the cytoplasm in an unspliced, singly spliced, or fully spliced form (13). The Rev protein, which is expressed from a fully spliced viral mRNA, was initially found to be absolutely required for expression of the viral structural proteins encoded by the various incompletely spliced viral mRNAs (67). Subsequently, it became apparent that these viral mRNAs, although expressed, were unable to reach the cytoplasm in the absence of Rev due to the nuclear retention mechanism mentioned above (41). However, nuclear export of these viral mRNAs could be induced by the combined action of Rev and a _cis_-acting structured viral RNA target, the Rev response element (RRE), that serves as the binding site for multiple Rev molecules (17, 40, 41). Mutational analysis of Rev identified an arginine-rich sequence, which serves as both a nuclear localization signal (NLS) and a sequence-specific RNA binding domain, that is in turn flanked by sequences that mediate the essential multimerization of Rev on the RRE (39, 40). A critical leucine-rich motif, initially termed the Rev activation domain, was also found to be essential for Rev function and was suggested to act as a cofactor binding motif (39). Subsequently, this leucine-rich sequence was shown to function as an autonomous nuclear export signal (NES) (16, 75) and it is now clear that leucine-rich NESs similar to the one found in Rev play a critical role in the appropriate subcellular localization of many cellular and viral proteins (21, 42, 50). NES sequences of the leucine-rich type were subsequently shown to serve as binding sites for Crm1, an essential nuclear export factor belonging to the Impβ family of nuclear transport receptors (18, 20, 53, 54, 68). In contrast, the Rev NLS serves as a binding site for Impβ itself (71). Nucleocytoplasmic shuttling by Rev, and nuclear export of bound HIV-1 mRNAs, therefore is dependent on the Ran-GTP gradient for both Rev import and Rev export, as indicated in Fig. 1.
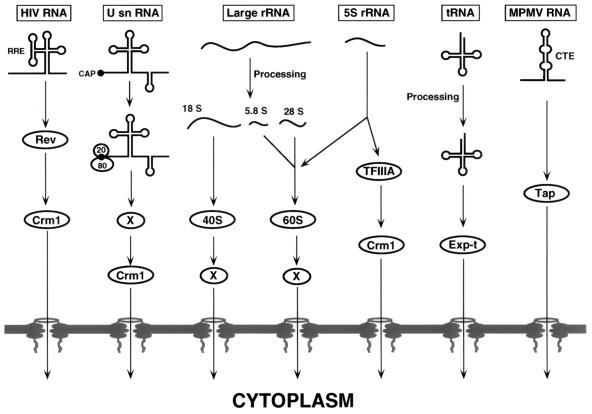
An important question was whether Crm1 would also mediate the nuclear export of other classes of cellular RNAs. Experiments designed to address this question, initially using competition assays with Xenopus oocytes and subsequently using reagents that specifically inhibit Crm1 function, have revealed that Crm1 is essential for U snRNA and 5S rRNA export but largely dispensable for cellular mRNA, tRNA, and large-rRNA export (8, 17, 52, 55) (Fig. 2).
FIG. 2.
Nuclear RNA export pathways. Shown is a schematic overview of several nuclear RNA export pathways used by vertebrate cells or by infecting retroviruses. RNA export is invariably mediated by one or more proteins, and these are indicated where known. Unknown, hypothetical export factors are indicated by the letter X. See the text for a detailed discussion.
NUCLEAR EXPORT OF U SNRNAS
The small nuclear ribonucleoprotein particles (snRNPs) are RNA-protein complexes that play a critical role in the splicing of nuclear pre-mRNAs. In higher eukaryotes, the snRNA components of the snRNPs are synthesized in the nucleus but, with the exception of U6, then assembled into mature snRNPs in the cytoplasm and reimported into the nucleus. The snRNAs that undergo this cytoplasmic assembly step, i.e., snRNAs U1, U2, U4, and U5, are synthesized by RNA polymerase II and, like mRNA, acquire a 7-methylguanosine (m7G) cap cotranscriptionally. This cap serves as the critical export signal for these U snRNAs (24).
The m7G cap is bound by the nuclear cap binding complex (CBC), consisting of the proteins CBP20 and CBP80, and CBC binding is required for U snRNA export (28) (Fig. 2). Because U snRNA export is mediated by Crm1, it initially seemed possible that CBC serves as a direct target for Crm1. However, neither CBP20 nor CBP80 contains a leucine-rich Crm1 binding motif and it therefore appears probable that U snRNA export involves an as yet unidentified NES-containing adapter protein (Fig. 2).
As noted above, Crm1 does not play a critical role in mRNA export although recruitment of Crm1 to the m7G cap may facilitate the export of very small mRNAs (29). The observation that Rev is unable to mediate viral mRNA export when its ability to multimerize on the RRE is inhibited (40) suggests that recruitment of multiple (>10) Rev, and hence Crm1, molecules may in fact be required to transport larger RNAs out of the nucleus to the cytoplasm (13). A single Crm1 molecule recruited to the cap of mRNA molecules could, however, serve to orient the mRNA during docking at the NPC, as mRNAs have been shown to exit the nucleus 5′ end first (14).
NUCLEAR EXPORT OF RRNAS
Three of the rRNAs (28S, 18S, and 5.8S) are transcribed in the nucleolus by RNA polymerase I as a single large pre-rRNA that is then processed, and extensively posttranscriptionally modified, to yield mature rRNAs species. The fourth rRNA, 5S rRNA, is transcribed by RNA polymerase III and requires only modest posttranscriptional processing. In a complex process, the 28S, 5.8S, and 5S rRNA species are then assembled, together with 50 ribosomal proteins, to form the 60S ribosomal subunit while the 18S rRNA is assembled with 33 ribosomal proteins to give the 40S ribosomal subunit (Fig. 2). These subunits are then separately exported to the cytoplasm by a mechanism that remains largely obscure. However, mutations in Ran, its regulators RanGAP and RanGEF, and certain nucleoporins do inhibit rRNA export in yeast cells (26, 47). It therefore seems possible that ribosomal subunit, and hence rRNA, export is dependent on one or more members of the Impβ family of nuclear transport receptors, although the inhibition in export observed upon disturbance of the Ran-GTP gradient could certainly be indirect, e.g., due to inhibition of the import of an rRNA export factor. Interestingly, both Crm1 and Exp-t, an Impβ family member important for tRNA export (see below), appear to be dispensable for ribosomal subunit export (26).
While 5S rRNA is normally assembled directly into large ribosomal subunits in the cell nucleus and therefore does not reach the cytoplasm on its own, an interesting exception exists in amphibian oocytes (51). In these highly specialized cells, an oocyte-specific type of 5S rRNA is expressed that then migrates to the cytoplasm, where it is stored pending the onset of vitellogenesis, when it is reimported into the nucleus and incorporated into 60S subunits. A major cytoplasmic 5S rRNA storage particle, the 7S particle, consists of 5S rRNA and the protein transcription factor IIIA (TFIIIA), which also plays a critical role in the transcription of 5S rRNA. Interestingly, amphibian TFIIIA contains a functional leucine-rich NES that is apparently lacking in mammalian TFIIIA (19). As noted above, nuclear export of 5S rRNA is specifically inhibited in Xenopus oocytes upon saturation of the Crm1 export pathway by nuclear injection of high levels of a leucine-rich NES peptide (16) and it therefore seems probable that, in this differentiated cell type, Crm1 also functions to mediate nuclear export of 7S storage particles containing TFIIIA and 5S rRNA (Fig. 2).
NUCLEAR EXPORT OF TRNAS
The formation of mature tRNAs from the pre-tRNAs generated by RNA polymerase III transcription requires a large number of posttranscriptional modification events, all of which occur in the nucleus (15). Specifically, all tRNAs are trimmed at the 5′ end by RNase P while U residues at the 3′ end are replaced by the CCA sequence found on all mature tRNAs. In addition, a large number of bases in the tRNA are modified while some pre-tRNAs also contain an intron in the anticodon loop that must be removed by splicing. Because detectable levels of pre-tRNAs do not normally reach the cytoplasm, it is apparent that the export mechanism used must be highly selective for mature tRNAs.
The first clue to the identity of the nuclear export factor for tRNAs was the identification of Los1p, an S. cerevisiae protein that markedly enhances the expression of a suppressor tRNA (25). However, because loss of Los1p function interfered with tRNA splicing, a role in tRNA export was not suspected until it became apparent that Los1p was a member of the Impβ family of nucleocytoplasmic transport factors. Cloning of the human homolog of Los1p, termed Exp-t, showed that Exp-t is able to bind directly and specifically to mature tRNA molecules but, as predicted in Fig. 1, only in the presence of Ran-GTP (1, 34). While the ability of Exp-t to bind to an RNA, rather than to a protein target, is unique among Impβ family members, Exp-t does share the ability of other Impβ family members to bind to specific nucleoporins and shuttle between the cell nucleus and cytoplasm.
A key prediction for the tRNA export factor is that it should be specific only for mature tRNAs. In fact, Exp-t binds very poorly to tRNAs that lack an appropriate 5′ or 3′ end or are not appropriately modified (2, 36, 37). Unexpectedly, Exp-t does bind to tRNAs that retain an intron and can export these from injected oocyte nuclei (2, 36). It has, however, been suggested that intron removal normally occurs prior to 5′- and 3′-end processing at physiological levels of tRNA expression (37), so this may not normally present a problem. It has also been suggested that Exp-t selectively binds to and exports tRNAs that have been aminoacylated in the nucleus (37, 62), although this hypothesis remains controversial (2, 36). Because aminoacylation would only occur effectively with mature tRNAs, this would, however, represent a highly effective quality control step prior to export.
NUCLEAR EXPORT OF MRNAS
Vertebrate mRNAs are generally produced as large precursor molecules that contain several, sometimes many, introns. As noted above, these introns must be specifically removed prior to nuclear export and translation. In addition to splicing, which for most mRNAs is largely cotranscriptional, mRNAs are also modified by the cotranscriptional addition of an m7G cap at the 5′ end and by the cleavage and polyadenylation of the 3′ end. Although both the cap and the poly(A) tail may modestly enhance mRNA export, neither is either necessary or sufficient (29).
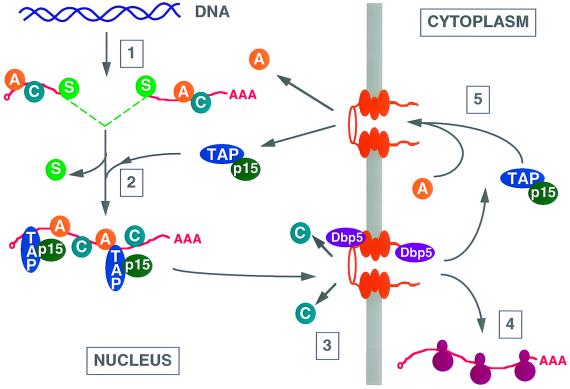
During transcription, pre-mRNAs are bound not only by splicing factors but also by members of the heterogeneous nuclear ribonucleoprotein (hnRNP) family of proteins (Fig. 3). These highly abundant proteins, which exist in 20 major forms, are thought to modulate many aspects of the nuclear fate of pre-mRNAs, including their appropriate processing and folding, and remain associated with nuclear mRNAs after splicing is completed (49, 50). During mRNA nuclear export, certain hnRNPs, e.g., hnRNP C, are selectively removed at the NPC while others, such as hnRNPs A1 and K, accompany the mature mRNA into the cell cytoplasm and are only then released and returned to the nucleus (42, 46, 53, 69) (Fig. 3). Analysis of the functional-domain organization of hnRNP A1 identified a sequence, termed M9, that could function as both an NLS and an NES (7, 44), thus raising the possibility that shuttling hnRNPs are directly involved in mediating nuclear mRNA export. It was therefore possible to envision a simple scenario in which pre-mRNAs are retained in the nucleus by splicing commitment factors, perhaps acting in concert with nonshuttling hnRNPs, until mRNA processing is completed. At this stage, the splicing factors would be released and the mature mRNA would then leave the nucleus under the direction of shuttling hnRNPs, such as hnRNPs A1 and K, concomitantly with loss of the nonshuttling hnRNPs. In this hypothesis, it is nuclear retention of pre-mRNAs that is active while mature mRNA export is essentially a default process that occurs once retention is released.
FIG. 3.
Model of vertebrate mRNA export. Pre-mRNA molecules are transcribed by RNA polymerase II (step 1) and rapidly assembled into RNPs containing several hnRNPs (indicated by the letter A or C) and splicing commitment factors (S). These pre-mRNAs cannot access nuclear RNA export factors (unless they contain a retroviral RNA export signal) and are retained in the nucleus. However, after completion of splicing, commitment factors are released and, by an unknown mechanism, nuclear mRNA export factors, including Tap and its cofactor p15, are recruited to the resultant mature mRNA (step 2). The RNP containing the mature mRNA then docks at the NPC (step 3) and exits the nucleus. At this time, nonshuttling hnRNPs, such as hnRNP C (C), are released and this may require the action of the essential NPC-associated RNA helicase Dbp5. Once in the cytoplasm, the mRNA is bound by cytoplasmic RNA binding proteins and used for translation (step 4) while the shuttling hnRNPs, including hnRNP A1 (A), as well as nuclear mRNA export factors, including Tap and p15, are released and recycled to the nucleus (step 5).
While it certainly remains probable that hnRNPs play a key role in nuclear mRNA export, this type of simple scenario now seems less plausible. For example, the hnRNP A1 M9 NES-NLS has been shown to be inaccessible on fully assembled mRNA-containing RNPs, thus making a critical role in mRNA export unlikely (65). Further, recent data generated with Xenopus oocytes have revealed that assembly of a mature mRNA into an hnRNP-containing RNP is not sufficient for nuclear export (38). Specifically, it was demonstrated that microinjection of a fully spliced synthetic mRNA into the oocyte nucleus, either naked or after assembly into RNPs in a nuclear extract, did not lead to effective nuclear export. In contrast, microinjection of the naked, pre-mRNA form of the same mRNA, or microinjection of an RNP containing the mature mRNA, generated during in vitro splicing, led to efficient nuclear export (38). Therefore, it is probable that efficient nuclear export requires recruitment of one or more factors that can be selectively assembled onto mRNAs during splicing and that are, in any event, only found on mature mRNA species (Fig. 3). It is important to note that splicing is not necessarily a prerequisite for nuclear mRNA export, as many mRNAs lacking introns can be efficiently expressed in vertebrate cells, while only a small percentage of yeast genes contain introns. Nevertheless, in the case of microinjected synthetic mRNAs, splicing does clearly enhance the efficiency of nuclear export.
If hnRNPs are not the primary export factor(s) for mRNAs, then what is? A strong candidate as a major mRNA export factor has recently emerged from a convergence of data obtained with yeast cells and from study of the retrovirus Mason-Pfizer monkey virus (MPMV). MPMV is a simple retrovirus; i.e., unlike HIV-1, MPMV only encodes structural proteins and therefore has no Rev equivalent (13). However, MPMV, like other retroviruses, faces the same problem of how to express both spliced and unspliced forms of the same initial transcript in the cell cytoplasm. In MPMV, this problem is solved by the viral constitutive transport element (CTE), a structured RNA element that induces unspliced viral mRNA export from infected cell nuclei (10). While functionally analogous to the HIV-1 RRE, the MPMV CTE is distinct in that its target protein must clearly be of cellular origin. Competition and inhibitor experiments subsequently revealed that MPMV CTE function also differs from the Rev-RRE system in that CTE function is not dependent on the Crm1 nuclear export factor (8, 55, 56, 60). These experiments further demonstrated that microinjection of high levels of the CTE into Xenopus oocyte nuclei selectively inhibited mRNA export, while tRNA, U snRNA, and Rev-RRE-dependent RNA export was unaffected (56, 60). This finding strongly suggested that the CTE cofactor was likely to be a key participant in cellular mRNA export.
Efforts to identify a cellular cofactor for the MPMV CTE led to the isolation of a protein, termed Tap, that was shown to bind specifically to functional forms of the MPMV CTE and to shuttle between the cell nucleus and cytoplasm (23, 31) (Fig. 2). Expression of human Tap enhances CTE function in microinjected oocytes and can effectively rescue CTE function in otherwise nonpermissive quail cells (23, 31). Mutational analysis of Tap soon defined the CTE RNA binding motif (9, 30) and also identified an essential domain, located at the Tap carboxy terminus, that functioned as a nucleocytoplasmic shuttle domain and that directly and specifically bound to several nucleoporins (3, 30, 32). These latter activities have proven to be mutationally inseparable, thus strongly arguing that the ability of Tap to exit the nucleus, and hence to export CTE-containing RNAs, is mediated by a direct interaction of Tap with constituents of the NPC (30). Nuclear export of Tap is therefore distinct from, for example, Rev nuclear export in that it is independent of any member of the Impβ family of nuclear transport factors. Curiously, however, while the Tap nucleocytoplasmic shuttling domain can function as both an NLS and an NES, Tap was found to contain an additional NLS that binds to transportin, a nuclear import factor belonging to the Impβ family (3, 72). Presumably, this second NLS, which is not essential for Tap function, simply promotes the rapid and efficient recycling of Tap to the cell nucleus.
Coincident with the above-described research on MPMV CTE function in vertebrate cells, the yeast homolog of Tap, termed Mex67p, was identified in a genetic screen and shown to be essential for nuclear export of total poly(A)+ RNA (64). Mex67p was found to directly interact in vivo with a second essential yeast mRNA export factor, termed Mtr2p, which promoted the efficient recruitment of Mex67p to yeast NPCs (61). Importantly, the Mex67p-Mtr2p heterodimer was found to directly bind to poly(A)+ RNA both in vivo and in vitro.
The search for the human protein equivalent to Mtr2p led to the identification of p15, which actually has little or no homology to Mtr2p (32). Nevertheless, p15 seems to be the functional homolog of Mtr2p as yeast cells lacking Mex67p can be complemented by human Tap plus p15 but not by Tap alone. In fact, Tap and p15 can rescue the viability of yeast cells lacking both Mex67p and Mtr2p, both of which are otherwise essential (32). This result demonstrates that the Tap-Mex67p nuclear RNA export pathway has been conserved through much of eukaryotic evolution and strongly suggests that p15 plays a critical role in the Tap-dependent export of cellular mRNA (Fig. 3).
So far, it has been difficult to define the role of p15 in the vertebrate cell, in part because p15 is not, in fact, essential for the Tap-dependent nuclear export of CTE-containing RNA (3, 30). However, p15 does enhance Tap binding to the CTE and can form a ternary complex with Tap on the CTE. However, p15 is clearly not essential for NPC binding and nucleocytoplasmic shuttling by Tap (3, 30). This finding raises the possibility that p15 normally acts at or near the final step in the mRNA export pathway by facilitating the recruitment of the Tap mRNA export factor exclusively to mature mRNAs (Fig. 3). The CTE, by recruiting Tap directly, may avoid this p15-dependent quality control step and hence induce the nuclear export of an unspliced viral mRNA whose access to Tap would otherwise be blocked.
Key questions in the area of nuclear mRNA export that remain unanswered include the identity of the factor(s) that mediates recruitment of the Tap-p15 heterodimer to mature mRNAs and, of course, whether other autonomous mRNA export pathways also exist. Several proteins have been reported that may play critical roles in the Tap-Mex67p-dependent mRNA export pathway or perhaps in other export pathways. One such protein is termed Gle2p in S. cerevisiae and Rae1 in human cells and in the yeast Schizosaccharomyces pombe. In S. pombe, Rae1 is essential for poly(A)+ RNA export from the nucleus and this deficiency can be complemented by the human Rae1 protein, again implying strong evolutionary conservation (4). Yeast Gle2p is normally associated with the nuclear pore (48), but evidence has been presented that human Rae1, while predominantly NPC associated, is also a nucleocytoplasmic shuttle protein that can associate with poly(A)+ RNA in vivo (33, 58). The role played by Rae1 in nuclear mRNA export is, however, unclear.
A second interesting protein, termed Dbp5, is required for poly(A)+ RNA export from the nucleus in both yeast and vertebrates (63, 66, 73). Dbp5 is a DEAD box type of helicase that is normally tightly associated with the cytoplasmic face of the NPC (73). However, Dbp5 can enter the nucleus and become trapped there in yeast cells lacking functional Crm1 or Mex67p (66), thus suggesting that Dpb5 normally shuttles in and out of the nucleus. Because the helicase activity of Dbp5 is essential for its biological activity, Dbp5 may function in the unwinding of mRNAs shortly before, during, and/or shortly after their traversal of the NPC (Fig. 3). In this way, Dbp5 might, for example, play a key role in the specific removal of nonshuttling hnRNPs, such as hnRNP C, at the NPC and also facilitate the entry of large, mRNA-containing RNPs into the central channel of the NPC.
PERSPECTIVE
While extraordinary progress has been made in the last 4 years in understanding the mechanisms underlying nuclear RNA export, significant questions remain unanswered. For example, the mechanism(s) underlying the nuclear export of ribosomal subunits is entirely unclear although recent progress with the yeast system (26, 47) suggests that this will not remain the case much longer. The complex mechanism(s) underlying mRNA export from the nucleus is also not fully understood. However, the possibility clearly exists that mRNA export is unique in that it may not involve any member of the Impβ family of nuclear transport receptors. Instead, it seems increasingly likely that mRNA export is mediated by an entirely distinct set of factors, such as Tap-Mex67p and Rae1-Gle2p, that nevertheless also exhibit the ability to bind to both cargo RNA molecules and components of the NPC. Clearly, for all RNA export pathways, how docking at the NPC occurs and how movement through the NPC and release at the cytoplasmic side of the nuclear membrane is regulated remain largely obscure. It seems likely that this particular problem will pose a challenge for several years to come.
One perhaps unexpected lesson that has emerged from the study of nuclear RNA export is the fact that incompletely or inappropriately processed RNAs are frequently poor substrates for the nuclear RNA export machinery, i.e., that the cell uses nuclear RNA export as a stringent quality control step to ensure that only functional RNAs reach the cytoplasm. This is most clearly apparent in the case of tRNA and mRNA export but may also be the case for other RNAs, such as rRNAs. How incompletely processed mRNAs are selectively retained in the nucleus and, conversely, how the export machinery can recognize when an mRNA is ready for nuclear export, remain particularly fascinating unresolved issues.
REFERENCES
- 1.Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 2.Arts G J, Kuersten S, Romby P, Ehresmann B, Mattaj I W. The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998;17:7430–7441. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachi A, Braun I C, Rodrigues J P, Panté N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Görlich D, Carmo-Fonseca M, Izaurralde E. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA. 2000;6:136–158. doi: 10.1017/s1355838200991994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharathi A, Ghosh A, Whalen W A, Yoon J H, Pu R, Dasso M, Dhar R. The human RAE1 gene is a functional homologue of Schizosaccharomyces pombe rae1 gene involved in nuclear export of poly(A)+ RNA. Gene. 1997;198:251–258. doi: 10.1016/s0378-1119(97)00322-3. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff F R, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear ras-related Ran. Proc Natl Acad Sci USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff F R, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- 7.Bogerd H P, Benson R E, Truant R, Herold A, Phingbodhipakkiya M, Cullen B R. Definition of a consensus transportin-specific nucleocytoplasmic transport signal. J Biol Chem. 1999;274:9771–9777. doi: 10.1074/jbc.274.14.9771. [DOI] [PubMed] [Google Scholar]
- 8.Bogerd H P, Echarri A, Ross T M, Cullen B R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J Virol. 1998;72:8627–8635. doi: 10.1128/jvi.72.11.8627-8635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun I C, Rohrbach E, Schmitt C, Izaurralde E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 1999;18:1953–1965. doi: 10.1093/emboj/18.7.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjöld M-L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breeuwer M, Goldfarb D. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990;60:999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- 12.Chang D D, Sharp P A. Regulation by HIV depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 13.Cullen B R. Retroviruses as model systems for the study of nuclear RNA export pathways. Virology. 1998;249:203–210. doi: 10.1006/viro.1998.9331. [DOI] [PubMed] [Google Scholar]
- 14.Daneholt B. A look at messenger RNP moving through the nuclear pore. Cell. 1997;88:585–588. doi: 10.1016/s0092-8674(00)81900-5. [DOI] [PubMed] [Google Scholar]
- 15.De Robertis E M, Black P, Nishikura K. Intranuclear location of the tRNA splicing enzymes. Cell. 1981;23:89–93. doi: 10.1016/0092-8674(81)90273-7. [DOI] [PubMed] [Google Scholar]
- 16.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 17.Fischer U, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornerod M, Ohno M, Yoshida M, Mattaj I W. Crm1 is an export receptor for leucine rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 19.Fridell R A, Fischer U, Lührmann R, Meyer B E, Meinkoth J L, Malim M H, Cullen B R. Amphibian transcription factor IIIA proteins contain a sequence element functionally equivalent to the nuclear export signal of human immunodeficiency virus type 1 Rev. Proc Natl Acad Sci USA. 1996;93:2936–2940. doi: 10.1073/pnas.93.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 21.Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 22.Görlich D, Pante N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 23.Grüter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 24.Hamm J, Mattaj I W. Monomethylated cap structures facilitate RNA export from the nucleus. Cell. 1990;63:109–118. doi: 10.1016/0092-8674(90)90292-m. [DOI] [PubMed] [Google Scholar]
- 25.Hopper A K, Schultz L D, Shapiro R A. Processing of intervening sequences: a new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell. 1980;19:741–751. doi: 10.1016/s0092-8674(80)80050-x. [DOI] [PubMed] [Google Scholar]
- 26.Hurt E, Hannus S, Schmelzl B, Lau D, Tollervey D, Simos G. A novel in vivo assay reveals inhibition of ribosomal nuclear export in Ran-cycle and nucleoporin mutants. J Cell Biol. 1999;144:389–401. doi: 10.1083/jcb.144.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj I W. A cap-binding protein complex mediating U sn-RNA export. Nature. 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- 29.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, Y., H. P. Bogerd, and B. R. Cullen. The biological activity of the human Tap nuclear RNA export factor is dependent on specific protein:protein interactions. J. Virol., in press.
- 31.Kang Y, Cullen B R. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 1999;13:1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung J U, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraemer D, Blobel G. mRNA binding protein mrnp 41 localizes to both nucleus and cytoplasm. Proc Natl Acad Sci USA. 1997;94:9119–9124. doi: 10.1073/pnas.94.17.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 35.Legrain P, Rosbash M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 36.Lipowsky G, Bischoff F R, Izaurralde E, Kutay U, Schafer S, Gross H J, Beier H. Coordination of tRNA nuclear export with processing of tRNA. RNA. 1999;5:539–549. doi: 10.1017/s1355838299982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund E, Dahlberg J E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science. 1998;282:2082–2085. doi: 10.1126/science.282.5396.2082. [DOI] [PubMed] [Google Scholar]
- 38.Luo M-J, Reed R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci USA. 1999;96:14937–14942. doi: 10.1073/pnas.96.26.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malim M H, Bohnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator—derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 40.Malim M H, Cullen B R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991;65:241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- 41.Malim M H, Hauber J, Le S-Y, Maizel J V, Cullen B R. The HIV-1 Rev transactivator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 42.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 43.Melchior F, Weber K, Gerke V. A functional homologue of the RNA1 gene product in Schizosaccharomyces pombe: purification, biochemical characterization, and identification of a leucine-rich repeat motif. Mol Biol Cell. 1993;4:569–581. doi: 10.1091/mbc.4.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 45.Michael W M, Eder P, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore M S, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- 47.Moy T I, Silver P A. Nuclear export of the small ribosomal subunit requires the ran-GTPase cycle and certain nucleoporins. Genes Dev. 1999;13:2118–2133. doi: 10.1101/gad.13.16.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy R, Watkins J L, Wente S R. GLE2, a Saccharomyces cerevisiae homologue of the Schizosaccharomyces pombe export factor RAE1, is required for nuclear pore complex structure and function. Mol Biol Cell. 1996;7:1921–1937. doi: 10.1091/mbc.7.12.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakielny S, Dreyfuss G. The hnRNP C proteins contain a nuclear retention sequence that can override nuclear export signals. J Cell Biol. 1996;134:1365–1373. doi: 10.1083/jcb.134.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 51.Nakielny S, Fischer U, Michael W M, Dreyfuss G. RNA transport. Annu Rev Neurosci. 1997;20:269–301. doi: 10.1146/annurev.neuro.20.1.269. [DOI] [PubMed] [Google Scholar]
- 52.Neville M, Rosbash M. The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae. EMBO J. 1999;18:3746–3756. doi: 10.1093/emboj/18.13.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 54.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 55.Otero G C, Harris M E, Donello J E, Hope T J. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J Virol. 1998;72:7593–7597. doi: 10.1128/jvi.72.9.7593-7597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjöld M-L, Dahlberg J E. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piñol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 58.Pritchard C E J, Fornerod M, Kapser L H, van Deursen J M A. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like Nup98 motif at the nuclear pore complex through multiple domains. J Cell Biol. 1999;145:237–254. doi: 10.1083/jcb.145.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 60.Saavedra C, Felber B, Izaurralde E. The simian retrovirus-1 constitutive transport element, unlike the HIV-1 RRE, uses factors required for cellular mRNA export. Curr Biol. 1997;7:619–628. doi: 10.1016/s0960-9822(06)00288-0. [DOI] [PubMed] [Google Scholar]
- 61.Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol. 1998;18:6826–6838. doi: 10.1128/mcb.18.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarkar S, Azad A K, Hopper A K. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNA in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96:14366–14371. doi: 10.1073/pnas.96.25.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitt C, von Kobbe C, Bachi A, Panté N, Rodrigues J P, Boscheron C, Rigaut G, Wilm M, Séraphin B, Carmo-Fonseca M, Izaurralde E. Dbp5, a DEAD box-protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J. 1999;18:4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Segref A, Sharma K, Doye V, Hellwig A, Huber J, Lührmann R, Hurt E. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siomi M C, Eder P S, Kataoka N, Wan L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snay-Hodge C A, Colot H V, Goldstein A L, Cole C N. Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J. 1998;17:2663–2676. doi: 10.1093/emboj/17.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sodroski J, Goh W C, Rosen C, Dayton A, Terwilliger E, Haseltine W. A second post-transcriptional transactivator gene required for HTLV-III replication. Nature. 1986;321:412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- 68.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 69.Stoffler D, Fahrenkrog B, Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr Opin Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- 70.Stutz F, Rosbash M. Nuclear RNA export. Genes Dev. 1998;11:2857–2868. doi: 10.1101/gad.12.21.3303. [DOI] [PubMed] [Google Scholar]
- 71.Truant R, Cullen B R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truant R, Kang Y, Cullen B R. The human Tap nuclear RNA export factor contains a novel transportin-dependent nuclear localization signal that lacks nuclear export signal function. J Biol Chem. 1999;274:32167–32171. doi: 10.1074/jbc.274.45.32167. [DOI] [PubMed] [Google Scholar]
- 73.Tseng S S, Weaver P L, Liu Y, Hitomi M, Tartakoff A M, Chang T H. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visa N, Alzhanova-Ericsson A T, Sun X, Kiseleva E, Bjorkroth B, Wurtz T, Daneholt B. A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell. 1996;84:253–264. doi: 10.1016/s0092-8674(00)80980-0. [DOI] [PubMed] [Google Scholar]
- 75.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 76.Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carrier-mediated translocation process. Proc Natl Acad Sci USA. 1983;80:6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]