Eukaryotic Translation Initiation Factor 4GI Is a Cellular Target for NS1 Protein, a Translational Activator of Influenza Virus (original) (raw)
Abstract
Influenza virus NS1 protein is an RNA-binding protein whose expression alters several posttranscriptional regulatory processes, like polyadenylation, splicing, and nucleocytoplasmic transport of cellular mRNAs. In addition, NS1 protein enhances the translational rate of viral, but not cellular, mRNAs. To characterize this effect, we looked for targets of NS1 influenza virus protein among cellular translation factors. We found that NS1 coimmunoprecipitates with eukaryotic initiation factor 4GI (eIF4GI), the large subunit of the cap-binding complex eIF4F, either in influenza virus-infected cells or in cells transfected with NS1 cDNA. Affinity chromatography studies using a purified His-NS1 protein-containing matrix showed that the fusion protein pulls down endogenous eIF4GI from COS-1 cells and labeled eIF4GI translated in vitro, but not the eIF4E subunit of the eIF4F factor. Similar in vitro binding experiments with eIF4GI deletion mutants indicated that the NS1-binding domain of eIF4GI is located between residues 157 and 550, in a region where no other component of the translational machinery is known to interact. Moreover, using overlay assays and pull-down experiments, we showed that NS1 and eIF4GI proteins interact directly, in an RNA-independent manner. Mapping of the eIF4GI-binding domain in the NS1 protein indicated that the first 113 N-terminal amino acids of the protein, but not the first 81, are sufficient to bind eIF4GI. The first of these mutants has been previously shown to act as a translational enhancer, while the second is defective in this activity. Collectively, these and previously published data suggest a model where NS1 recruits eIF4GI specifically to the 5′ untranslated region (5′ UTR) of the viral mRNA, allowing for the preferential translation of the influenza virus messengers.
Upon infecting a host, influenza virus efficiently shuts off host cell gene expression (75). Consequently, the virus has evolved subtle strategies to ensure the selective and efficient expression of its genes. These include a decreased synthesis and/or degradation of cellular mRNAs, probably as the consequence of the virus-induced cap-snatching activity, inhibition of nucleocytoplasmic transport of mRNA (28), and cytoplasmic degradation of cellular mRNAs (1, 22). The cellular protein synthesis machinery is kept competent during influenza virus infection by avoiding the activation of the double-stranded RNA-activated kinase (PKR) (37, 38, 41, 61, 78). Viral RNA messengers bear a capped 5′ untranslated region with a highly conserved sequence common to all genes. The 3′ terminus is polyadenylated by reiterative copy of a U5–7 track present near the 5′ end of the viral RNA (42, 62, 63, 70). Therefore, viral mRNAs are formally equivalent to cellular mRNAs (31, 33). Nevertheless, influenza virus inhibits cellular mRNA translation at both initiation and elongation steps (27), while it selectively enhances viral mRNA translation, with the sequences contained within the 5′ untranslated region (5′ UTR) playing a critical role (14).
Among the virus gene products, NS1 is the only nonstructural protein (34). It accumulates in the nucleus early in the infection and in the nucleus and the cytoplasm at later times (52). A fraction of the protein has been found in association with polysomes (3, 6, 32). NS1 is an RNA-binding protein that binds viral vRNA, the 5′ untranslated specific region of viral mRNAs, double-stranded RNA, U6 snRNA, and poly(A)-containing mRNA, (16, 19, 45, 59, 66, 67). When expressed from cDNA, the protein behaves as a posttranscriptional modulator, altering pre-mRNA splicing (9, 40) and inhibiting cellular mRNA polyadenylation (50) and poly(A)-containing mRNA nucleocytoplasmic transport (9, 66). In addition, NS1 stimulates viral protein synthesis (3, 5) by increasing the rate of initiation of viral mRNA translation (3). This enhancement is dependent on the presence of sequences at the 5′ UTR of the mRNAs (3).
In eukaryotic cells, most of the processes relative to translational control affect the initiation stage. This step involves the cap-dependent assembly of the preinitiation complex at the 5′ end of mRNA, a process that includes the activity of the eukaryotic initiation factor 4F (eIF4F) (a complex of the cap-binding protein eIF4E, the ATP-dependent helicase eIF4A, and the eIF4GI protein) and eIF3 (reviewed in references 48, 49 and 68). In influenza virus-infected cells, several of these initiation factors are altered. Thus, the cap-binding protein eIF4E is underphosphorylated and eIF4GI becomes hyperphosphorylated (7); moreover, influenza virus infection cannot proceed in poliovirus-infected cells, where eIF4GI is proteolytically cleaved (77). In view of these alterations of protein synthesis in influenza virus-infected cells and the involvement of NS1 protein in the specific enhancement of viral mRNA translation, we looked for cellular interaction targets for the influenza virus NS1 protein among translation initiation factors. Here we report that eIF4GI protein specifically interacts with NS1, both in vivo and in vitro, in an RNA-independent manner. Moreover, a correlation was found between the activity of NS1 deletion mutants as translational activators and their ability to interact with eIF4GI.
MATERIALS AND METHODS
Biological materials.
The COS-1 cell line (15) was obtained from Y. Gluzman, and the MDCK cell line was purchased from the American Type Culture Collection. Cell cultures were grown in Dulbecco's modified Eagle medium (DMEM) containing 5% fetal bovine serum. The influenza virus A/Victoria/3/75 strain was grown in MDCK cells as reported previously (57). Plasmid pGST-NS1 coding for NS1 as a fusion protein with glutathione _S_-transferase (GST) (18) was kindly provided by R. Fukuda. Plasmid pSK-HFC1, containing an N-terminally deleted cDNA sequence for the human eIF4GI gene that encodes a protein without the first 156 amino acids, was kindly provided by R. E. Rhoads. Plasmid pMV-7, containing the murine cDNA of eIF4E, and plasmid pCDNA3-HA-eIF4GI, containing the entire cDNA sequence of eIF4GI with a hemagglutinin epitope at the N terminus, were kindly provided by N. Sonenberg. Plasmids expressing influenza virus NS1 or NS2 protein (pSVa232NS1 or pSVa232NS2), as well as plasmids expressing deletion-carrying versions of the NS1 protein or a His-tagged NS1, have been previously described (9, 45). The preparation of antisera specific for NS1 protein has been reported (45, 52). Rabbit antiserum specific for the eIF4GI protein was prepared by immunizing animals with synthetic peptides coupled to keyhole limpet hemocyanin, corresponding to positions 192 to 212 and 1152 to 1177 of the human protein (54). These antibodies recognized the translation factor from both humans and primates (53). Monoclonal antibodies against human poly(A)-binding protein (PABP) were kindly provided by G. Dreyfuss.
Mutant construction.
To obtain eIF4GI deletion mutants, plasmid pSK-HFC1 (26) was digested with _Eco_RI and religated, and a clone was selected in which the cDNA was under the control of the T7 promoter instead of the T3 promoter. This new clone was named peIF-4GIΔ157. Digestion of peIF-4GIΔ157 plasmid with _Sma_I or with _Bam_HI, followed by autoligation, led to peIF-4GI157-550 or peIF-4GI157-614 plasmid, coding for residues 157 to 550 or 157 to 614 of the protein, respectively. Recombinant peIF4GI868-1133 coding for amino acids 868 to 1133 of the human eIF4GI protein was generated by PCR amplification using as template plasmid peIF-4GIΔ157 and the corresponding oligonucleotides. The primers used contained additional _Hin_cII and _Xba_I restriction sites at the N terminus and C terminus, respectively, allowing its insertion in plasmid Bluescript SK cut with _Hin_cII-_Xba_I. Recombinant pHis-eIF4GI157-550 was prepared as follows: first, we inserted an _Eco_RV fragment of peIF-4GIΔ157 plasmid, containing the 4GI coding sequence with an N-terminal deletion of 157 amino acids, into _Pvu_II-digested pRSETC plasmid (Invitrogen); and second, this pRSET recombinant was cut with _Sma_I and autoligated to generate the pHis-4GI157-550 construct, which encodes residues 157 to 550 of eIF4GI fused to a histidine tag.
NS1 deletion mutant pGNS1Δ1-80 was obtained by digesting the pGNS1 plasmid (45) with _Asp_718 at the plasmid polylinker and with _Nco_I endonuclease at the N-terminal coding sequence of the NS1 gene. Ligation of the digested plasmid was achieved by using a mixture of oligonucleotides that provides _Asp_718 and _Nco_I sites in the ligation, generating a plasmid that starts translation at position 81 in the NS1 coding sequence.
To produce plasmid pHis-PAΔ1-454, pGPA plasmid (74) was digested with the appropriate enzymes to get the coding sequence for the PA polymerase subunit from the influenza virus A/Victoria/3/75 strain. This coding sequence was ligated to pRSETA plasmid to obtain the pHis-PA plasmid that encodes the entire His-PA fusion protein. This plasmid was digested with _Sma_I and _Sca_I and autoligated. The obtained recombinant plasmid, pHis-PAΔ1-454, encodes a His-tagged deleted PA version where residues between 1 and 454 have been removed.
Cell transfection.
For transfection of COS-1 cells, a mixture of cationic liposomes and DNA (2 μl/μg of DNA) (71) was added to the cultures containing serum-free DMEM. They were incubated overnight, washed with phosphate-buffered saline (PBS), and refed with fresh DMEM containing 5% fetal calf serum. After 48 h of further incubation, the cell cultures were used for analysis.
Protein expression and purification.
The His-NS1, His-PAΔ1-464, and His-4GI157-550 proteins were expressed in Escherichia coli BL21DE3 pLysS cells harboring plasmid pRSHisNS1, pHis-PAΔ1-464, or pHis-eIF4GI157-550, respectively. The GST and GST-NS1 proteins were expressed in E. coli DH5 cells harboring plasmid pGEX-2T and pGEX-NS1, respectively. The GST and GST-NS1 proteins were purified according to the manufacturer's instructions (Pharmacia Biotechnology). For purification of His-NS1, His-PAΔ1-464, and His-4GI157-550, expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) (10 μM for His-NS1 protein or 1 mM for the other proteins) for 2 h at 30°C. The cells were resuspended in a buffer containing 50 mM Tris-HCl, 500 mM NaCl, 5 mM MgCl2, 10% glycerol, 0.1% NP-40, and 100 mM imidazol (pH 8.0) (supplemented before use with 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM tolylsulfonyl phenylalanyl chloromethyl ketone [TPCK], 1 mM _N_α-_p_-tosyl-l-lysine chloromethyl ketone [TLCK], and 10 mM 2β-mercaptoethanol [2ME]) and were sonicated. After removal of cell debris by centrifugation, the supernatant was incubated with Ni2+-nitrilotriacetic acid-agarose resin (Invitrogen) and equilibrated in the same buffer by rocking overnight at 4°C. After extensive washes with 20 mM Tris-HCl–1 M KCl (0.1 M KCl for eIF-4GI157-550 protein purification)–5 mM MgCl2–10% glycerol–10 mM 2ME–50 mM imidazole (pH 8.0) (washing buffer), the proteins were eluted with 1 M imidazole in washing buffer.
In vitro transcription-translation.
Plasmids encoding eIF-4GIΔ157 protein, NS1 protein, or mutants thereof were used for in vitro transcription-translation using the Promega TNT coupled system. In all cases, the genes were expressed under the T7 promoter and a 35S-labeled methionine-cysteine mixture (1,400 μCi/ml) was added to the cell-free protein synthesis system. After 2 h of incubation at 30°C, the mixture was centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were centrifuged again at 250,000 × g for 2 h at 4°C. The postribosomal supernatants were then used as a source of recombinant protein for in vitro binding studies.
Western blotting.
Western blottings were done as described previously (74). The following primary antibodies were used: for eIF4GI, a mixture of the rabbit antibodies against N-terminal or C-terminal peptides of eIF4GI (1/3,000 dilution each) was used, and for NS1 protein, a rabbit anti-NS1 serum prepared by hyperimmunization with His-NS1 protein (45) (1/300 dilution) was used. For His-tagged proteins, a rabbit anti-His peroxidase serum (Santa Cruz Biotechnology) (1/5,000 dilution) was used.
Coimmunoprecipitation.
Cultures of COS-1 cells were either mock infected, infected with influenza virus, or transfected with either pSVa232NS1 or pSVa232NS2 plasmid. In vivo protein labeling was done at 5 h or 8 h postinfection, as indicated in Results, or 60 h posttransfection by incubating cells with [35S]methionine-cysteine (Amersham) at 1,200 μCi/ml in methionine-cysteine-free DMEM for 1 h. Cells were washed with ice-cold PBS, scraped off the plates, and lysed in a buffer containing 150 mM NaCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.5), and 0.5% NP-40 (extraction buffer). The extracts were clarified by centrifugation at 10,000 × g for 15 min and were used for coimmunoprecipitation assays. The extracts were incubated with preformed protein A-Sepharose-immunoglobulin G complexes for 2 h at room temperature. The immunoprecipitates were washed four times with extraction buffer, boiled in Laemmli sample buffer, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, wild-type NS1 or deletion-containing versions of the protein were translated in vitro in the presence of [35S]methionine-cysteine and used for coimmunoprecipitation assays as described previously (64). Briefly, the in vitro translation extracts were diluted in a buffer containing 100 mM KCl, 1.5 mM MgCl2, 10 mM Tris (pH 7.5), and 0.4% NP-40 (binding buffer) and were preadsorbed with protein A-Sepharose. The supernatants were then incubated with the corresponding antibodies, and the immunoprecipitates were extensively washed with binding buffer and analyzed by SDS-PAGE.
Pull-down experiments.
For pull-down experiments using cell extracts, purified His-PAΔ1-454 and His-NS1 proteins were bound to fresh Ni2+-nitrilotriacetic acid-agarose as described above. eIF4GI was obtained from the cytoplasmic fraction of COS-1 cells and prepared as previously described (45). The extracts were diluted in a buffer containing 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.5 mM EDTA, 0.6% Nonidet P-40, 2 mM dithiothreitol, 50 mM imidazole or 100 mM imidazole when indicated, 1 mM PMSF, 1 mM TLCK, 1 mM TPCK, and 1 mM benzamidine (interaction buffer) plus 60 mM KCl (low salt buffer [LSB]) or 1 M KCl (high salt buffer [HSB]), as indicated in Results, and were centrifuged at 20,000 × g for 1 h at 4°C to obtain a postribosomal supernatant. These supernatants were incubated with His-NS1- or His-PAΔ1-454-containing resin or with an empty matrix in LSB or HSB, depending on the salt concentration of the postribosomal supernatant. After incubation for 1 h at room temperature, the resins were washed with the corresponding buffer and were eluted with 1 M imidazole in the same buffer. For pull-down assays with in vitro translated eIF4GI or eIF4E, the proteins were synthesized by transcription-translation of the corresponding cDNAs, and the binding assays with the NS1-containing matrix or with the empty matrix were carried out as described when cell extracts were used. For pull-down assays of eIF4GI deletion mutants or HA-eIF4GI protein, the proteins were synthesized by transcription-translation of the corresponding cDNAs, diluted in a buffer containing 150 mM NaCl, 10 mM Tris, 1.5 mM MgCl2, 0.1% Nonident P-40 (pH 8.5), and 50 mM imidazole and were centrifuged at 200,000 × g for 1 h at 4°C to obtain a postribosomal supernatant. After incubation for 2 h at room temperature, the resins were washed extensively in the same buffer, and the labeled proteins retained were analyzed by electrophoresis. For pull-down experiments of NS1 mutants with eIF4GI157-550 protein, the full-length or deletion-containing versions of NS1 were first translated in vitro, were diluted with an excess of interaction buffer without imidazole, and were applied to an empty matrix to remove the unspecific binding. The recovered NS1 proteins were applied to the 4GI157-550-containing matrix or to the empty matrix in high salt interaction buffer with 100 mM imidazole. After incubation for 2 h at room temperature, the resins were washed extensively, and the labeled proteins retained were analyzed by electrophoresis. For pull-down experiments with GST fusion proteins, GST and GST-NS1 proteins were purified as described above and were bound to Sepharose 4B-glutathione resins. Purified His-eIF4GI157-550 was added and incubated for 1 h at room temperature in a buffer containing 150 mM NaCl, 10 mM Tris, 1.5 mM MgCl2, and 0.5% Nonidet P-40 (pH 8.5). After the incubation, resins were washed three times with 10 volumes of the same buffer, and the bound protein was eluted with 10 mM glutathione and was analyzed by Western assays using specific antibodies.
Overlay assay.
As a direct protein-protein interaction test, the overlay technique was chosen (30). In brief, different amounts of recombinant proteins, or bovine serum albumin used as a control, were applied to a nitrocellulose filter equilibrated in PBS by using a dot blot apparatus. After protein adsorption, the filter was dried at 37°C for 1 h. The filter was then rehydrated in PBS and blocked with PBS plus 2% low-fat milk. The counterpart protein (His-NS1, His-4GI157-550, or protein A) was then added at a concentration of 5 ng/ml in PBS containing 2% low-fat milk and 10% sucrose and was incubated at room temperature for 1 h with shaking. The filter was washed three times for 10 min with PBS, and the retained protein was fixed with 0.5% formaldehyde in PBS for 1 h at room temperature. Fixation was stopped by incubating with 2% glycine in PBS for 1 h at room temperature and washing with PBS–0.05% Tween 20. Finally, a Western blot analysis was carried out using antibodies specific for the counterpart proteins, as already described. Where indicated, NS1 or eIFGI recombinant proteins were treated with RNase A (2 mg/mg of recombinant protein) for 30 min at 4°C.
RESULTS
NS1 protein associates in vivo with the eIF4GI subunit of eIF4F.
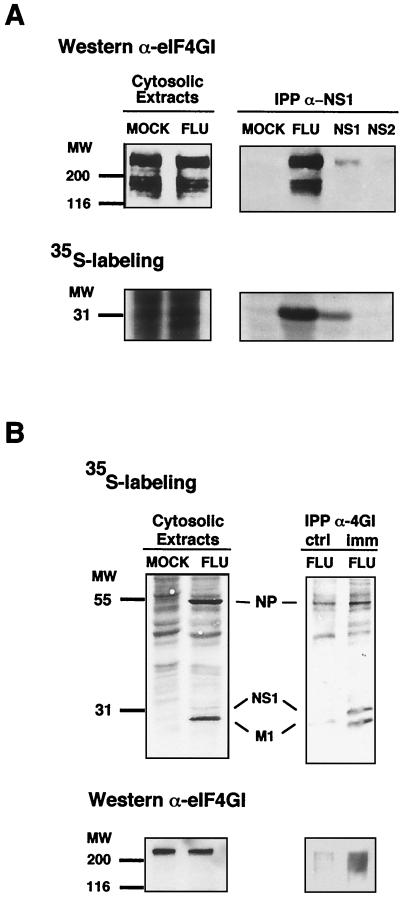
Previous results had shown that influenza virus NS1 protein expression led to an increase in the size of viral polysomes (3), indicating that NS1 stimulated translation by, at least, enhancing the rate of initiation. Therefore, we analyzed the possible interaction of NS1 protein with translation initiation factors that could mediate such activation. Since the eIF4E subunit of eIF4F is underphosphorylated (i.e., inactivated) during influenza virus infection (7), and translation of influenza virus mRNAs is inhibited in poliovirus-infected cells, in which the eIF4GI subunit of eIF4F is degraded (77), eIF4F was chosen as a possible NS1-binding translation initiation factor. The binding of eIF4GI with NS1 protein was studied first. Cultures of COS-1 cells were either infected with influenza virus, mock transfected, or transfected with plasmids expressing NS1 (pSVa232NS1) or NS2 (pSVa232NS2) protein. The cultures were metabolically labeled with [35S]methionine-cysteine, and cytoplasmic extracts were immunoprecipitated with anti-NS1 serum or control serum. The immunoprecipitates, as well as samples of total cell extracts, were resolved by SDS-PAGE and were transferred to a nitrocellulose filter. The presence of eIF4GI in the immunoprecipitates was detected by Western blot analysis (Fig. 1A, upper panels), and that of NS1 protein was detected by autoradiography (Fig. 1A, lower panels). The results show that the amount of eIF4GI in total cell extracts was not affected by influenza virus infection (Fig. 1A, upper left panel). Using NS1-specific antibodies, around 10% of the eIF4GI was coimmunoprecipitated from extracts of influenza virus-infected cells, and this protein was also present in the anti-NS1 immunoprecipitates of COS-1 cells expressing NS1 protein (Fig. 1A, upper right panel). In contrast, eIF4GI protein was not detected in anti-NS1 immunoprecipitates derived from mock transfected cells or cells transfected with a plasmid expressing NS2 as a control (Fig. 1A, upper right panel). Quantitative determinations of eIF4GI signals (Western blot) and NS1 protein signals (autoradiography) in the immunoprecipitates indicated that the eIF4GI/NS1 ratio was around three times higher in cells infected with influenza virus than in cells transfected with NS1-expressing plasmid (data not shown). These results indicate that NS1 can be found in a complex with endogenous eIF4GI during influenza virus infection and that complex formation is not dependent on other influenza virus products. As influenza virus NS1 protein is an RNA-binding factor able to interact with several proteins, we asked whether NS1 could be coimmunoprecipitated from in vivo labeled influenza virus-infected cells using anti-4GI antibodies. For this purpose, we used cytosolic extracts of virus-infected cells, in which the amount of NS1 was low in comparison with other, abundant viral proteins like NP or M1 (see labeled proteins in Fig. 1B, upper left panel). Coimmunoprecipitation assays were carried out with preimmune or immune anti-4GI serum (Fig. 1B, ctrl or imm, respectively), and the presence of eIF4GI protein was checked by Western blotting (Fig. 1B, bottom panels). The results showed that NS1 protein was clearly enriched in the immunoprecipitation carried out with anti-4GI antibody, compared to other influenza virus RNA-binding proteins like NP and M1.
FIG. 1.
In vivo association of NS1 to eIF4GI. Cultures of COS-1 cells were infected with influenza virus A/Victoria/3/75 (FLU), transfected with plasmid pSVa232NS1 (NS1) or pSVa232NS2 (NS2), or mock transfected (MOCK). The cultures were labeled with [35S]methionine-cysteine (35S-labeling), and cytoplasmic extracts were prepared at 5 h postinfection (A), 8 h postinfection (B), or 60 h posttransfection. (A) Shown are cytosolic extracts immunoprecipitated with anti-NS1 (IPP α-NS1) and the immunoprecipitates and samples of the extracts prior to immunoprecipitation (Cytosolic Extracts), separated in polyacrylamide-SDS gels. The gels were blotted to nitrocellulose and exposed for autoradiography. Finally, the filters were developed in a Western assay using anti-eIF4GI serum. (B) Cytosolic extracts were immunoprecipitated with either preimmune (ctrl) or anti-4GI serum (imm) and separated in polyacrylamide-SDS gels. The gels were dried and exposed for autoradiography (upper panels) or blotted to nitrocellulose and developed for Western assay using anti-eIF4GI serum (lower panels).
NS1 pulls down 4GI protein but not the 4E subunit of eIF4F.
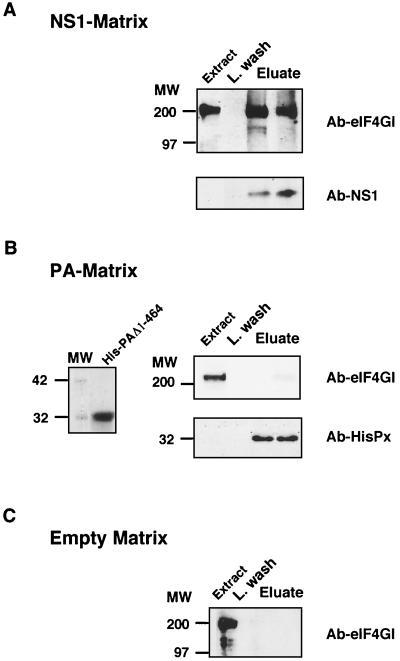
To further characterize the association of NS1 and eIF4GI protein, we carried out in vitro binding studies with purified His-NS1 protein. Resins containing either a deletion-containing version of the influenza virus PA polymerase subunit as a histidine-tagged protein or an empty matrix were used as negative controls. Analysis of the purified His-NS1 preparation used showed an apparent homogeneous protein (see Fig. 5A) as well as the purified His-PA protein that contains residues between 455 and 716 (Fig. 2B, left panel). The postribosomal supernatant of a cytoplasmic fraction of COS-1 cells, prepared in HSB as indicated in Materials and Methods, was used in binding assays with either His-NS1- or His-PAΔ1-464-containing resin or the empty matrix. The resins were washed with HSB and were eluted with HSB containing 1 M imidazole. The presence of eIF4GI, NS1, or PA protein was determined by Western blotting, and the results are presented in Fig. 2. As can be observed, 4GI protein was specifically detected in the eluate of the His-NS1 matrix (Fig. 2A, upper panel) but not in the His-PA eluate (Fig. 2B, upper right panel) or in the empty matrix eluate (Fig. 2C). To ascertain the presence of the His proteins in the eluates, we carried out Western analysis with specific antibodies. The results are presented in Fig. 2A and B, bottom (Ab-NS1 and Ab-HisPx, respectively), and they indicate the presence of both proteins in the corresponding eluates.
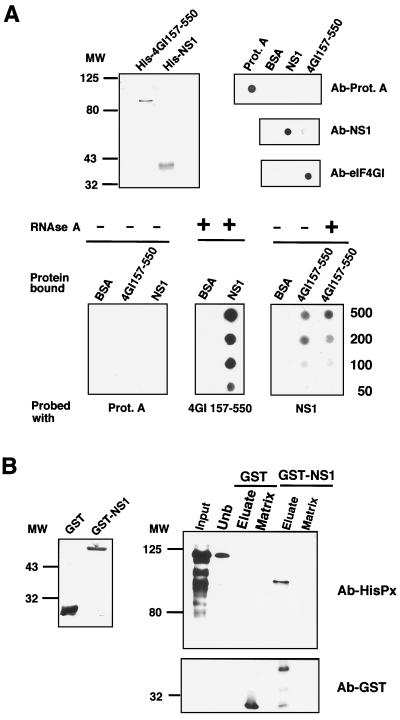
FIG. 5.
NS1 and eIF4GI interact directly. (A) Overlay experiments. The upper left panel shows the purification of His-eIF4GI157-550 and His-NS1 recombinant proteins. The proteins were purified by Ni2+-containing resins as described in Materials and Methods, analyzed by SDS-polyacrylamide gels, and stained with Coomassie blue. The upper right panel indicates the specificity of the antibodies. A total of 500 ng of protein A, BSA, purified His-eIF4GI157-550 (4GI157-550), or His-NS1 (NS1) protein was applied to nitrocellulose filters and developed with antibodies specific for NS1 or eIF4GI or with an anti-rabbit peroxidase for protein A detection. The lower panel represents an overlay assay. Different amounts (from 50 to 500 ng) of BSA or purified recombinant His-eIF4GI157-550 (4GI157-550) or His-NS1 (NS1) protein were applied to nitrocellulose filters (Protein bound). The filters were incubated with solutions containing either protein A, His-eIF4GI157-550, or His-NS1 at 5 ng/ml, were fixed with formaldehyde, and were extensively washed (Probed with). Finally, the binding of the probe protein was detected by Western blotting. Where indicated, both proteins used for binding were treated with RNase A. (B) Pull-down experiments. The left panel shows GST or GST-NS1 expressed and purified from bacteria as described in Materials and Methods. The right panel indicates His-eIF4GI157-550 protein incubated with GST- or GST-NS1-containing resin. After incubation and extensive washing, the retained protein was eluted with 10 mM glutathione. Samples of purified His-eIF4Gt157-550 (Input), unbound protein (Unb), eluate, and the corresponding matrix were analyzed by SDS-PAGE followed by Western assays with antibodies against His (Ab-HisPx) or GST (Ab-GST).
FIG. 2.
NS1 pulls down initiation factor eIF4GI. Cytoplasmic fractions of COS-1 cells were incubated with a His-NS1-containing matrix (A), a His-PAΔ1-464-containing matrix (B), or an empty matrix (C). After incubation for 1 h, resins were washed with HSB and the retained protein was eluted with 1 M imidazol (Eluate), as described in Materials and Methods. Fractions corresponding to cellular extract (Extract), the last wash (L. wash), and eluate were analyzed by SDS-PAGE, transferred to Immobilon (Millipore), and developed for Western assays with specific anti-eIF4GI, anti-NS1, or anti-HisPx antibodies. Ten percent of the input and 30% of the fractions eluted with 1 M imidazole were analyzed. The left side of panel B shows the purified His-PA deletion-containing protein used in the His-PA-containing matrix.
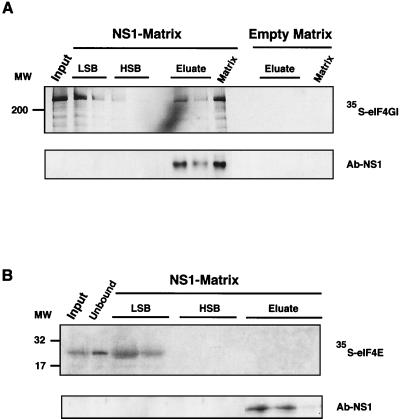
Similar experiments were carried out using the in vitro translated, labeled eIF4GI factor (eIF4GIΔ1-157) (26) (Fig. 3). In these experiments, the translation mixture was centrifuged in LSB (see Materials and Methods) to obtain a labeled eIF4GI protein largely free of endogenous protein which should remain associated with the preinitiation complexes (69). The supernatant was incubated with the empty matrix or the His-NS1-containing matrix in LSB, and after washing with LSB and HSB, the bound eIF4GI was eluted with 1 M imidazol. As can be observed (Fig. 3A), a fraction of the in vitro labeled eIF4GI remained bound to the His-NS1-containing column and was only eluted when the influenza virus protein was released. The amount of eIF4GI protein specifically retained by the NS1-containing matrix was around 20% of the total applied protein, either synthesized in vitro or obtained from COS-1 cells.
FIG. 3.
NS1 retains in vitro synthesized eIF4GI but not eIF4E. Plasmids peIF-4GIΔ156 and pMV-7 were used in an in vitro transcription-translation reaction in the presence of [35S]methionine-cysteine to generate eIF4GIΔ156 (A) and eIF4E (B) proteins, respectively. Postribosomal supernatants of the translation mixtures were applied to an NS1-containing matrix (NS1-Matrix) or to an empty matrix. After incubation for 1 h, resins were washed with LSB, HSB, and 1 M imidazole (Eluate). Total supernatant (Input), samples of the different fractions, and the remaining resins (Matrix) were analyzed by denaturing gels and exposed for autoradiography. Ten percent of the input and 30% of the other samples are shown in the figure. Fractions used in LSB and HSB represent the first and the last samples of these washing conditions. The same samples were analyzed for the presence of NS1 protein by Western assays with specific antibodies (Ab-NS1).
We next studied whether NS1 protein was able to bind the eIF4E subunit of the eIF4F complex. To that end, we labeled the eIF4E protein by in vitro transcription-translation and prepared a postribosomal supernatant in LSB buffer. The binding of labeled eIF4E to a His-NS1-containing matrix was studied as indicated above, and the results are presented in Fig. 3B. Labeled eIF4E protein was not retained, indicating that the cap-binding factor of the eIF4F complex and NS1 do not interact in vitro.
Mapping of the eIF4GI region that interacts with NS1 protein.
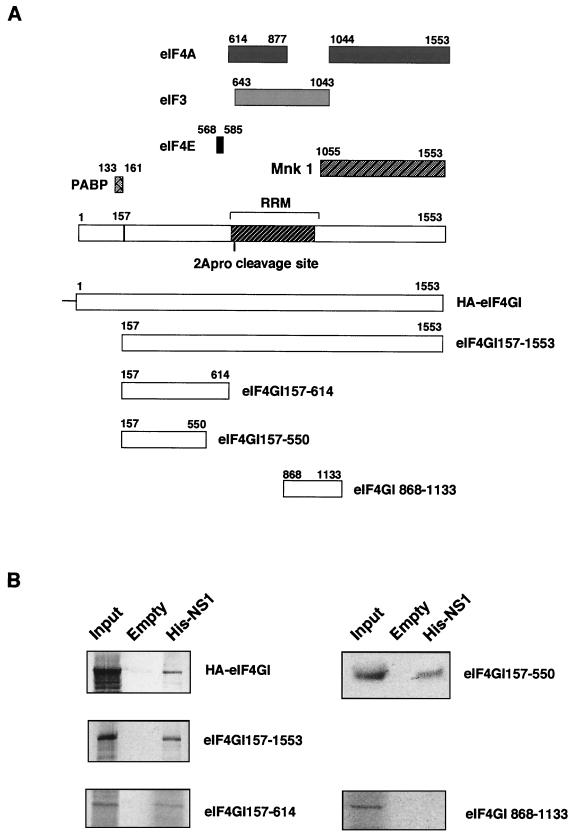
The eIF4GI subunit of eIF4F has been proposed to behave as a connector in the formation of the preinitiation complex (reviewed in references 49, 58, and 73). Thus, it binds a long list of proteins involved in this step of the initiation or its regulation: poly(A)-binding protein (21, 79), eIF4E (35, 43), eIF3 (35), ATP-dependent RNA helicase (eIF4A protein) (35), and mitogen-activated protein kinase Mnk1 (64) (see scheme in Fig. 4A). This property of eIF4GI prompted us to further examine if these eIF4GI-interacting proteins could mediate NS1-4GI association. Therefore, besides analyzing the behavior of the N-terminally truncated version of eIF4GI, we constructed some deletion mutants of eIF4GI protein and tested their ability to bind NS1 protein. One of these truncated 4GI proteins contains residues 157 to 614 (eIF4GI157-614) and lacks the regions interacting with eIF3, eIF4A, and Mnk1 proteins. The eIF4GI157-614 protein was labeled by in vitro transcription-translation, and its ability to bind a His-NS1-containing matrix was studied as indicated above. The results presented in Fig. 4B show that eIF4GI157-614 specifically interacts with the NS1-containing resin. These results suggest that eIF3, eIF4A, and Mnk1 proteins do not mediate the interaction between eIF4GI and NS1 proteins. To reevaluate the involvement of eIF4E as a possible mediator of NS1-4GI interaction, a new truncated version of the 4GI protein was constructed (mutant eIF4GI157-550) and was assayed for interaction with NS1 protein. This 4GI mutant contains amino acids 157 to 550 of the molecule and lacks the residues involved in eIF4E binding (positions 568 to 585). The results are presented in Fig. 4B. They clearly show that this deletion mutant is also able to bind NS1 and indicate that eIF4E protein is not required for eIFGI-NS1 interaction. Quantitation of the 4GI deletion mutants bound by the NS1 matrix indicated that around 10% of either protein was retained. Additional pull-down experiments were carried out using an eIF4GI deletion mutant containing amino acids 868 to 1133 (mutant eIF4GI868-1133) as a negative control and a recombinant plasmid expressing the entire eIF4GI coding sequence with a hemagglutinin epitope at the N terminus (HA-eIF4GI) as an extra positive control. Mutant eIF4GI868-1133 was unable to bind to His-NS1 containing resins, whereas recombinant HA-eIF4GI was positive (Fig. 4B). Collectively, these data indicate that the association of eIF4GI to NS1 protein is not mediated by any known eIF4GI-interacting protein, and the data localize the region of interaction within amino acids 157 to 550 of the molecule.
FIG. 4.
Mapping of eIF4GI-interacting domains. (A) Scheme of the eIF4GI regions involved in the interaction with translation-related proteins and of the eIF4GI proteins used in panel B. (B) The N-terminal domain of eIF4GI interacts with NS1. Postribosomal supernatants of in vitro labeled eIF4GI recombinants were applied to an NS1-containing matrix (His-NS1 matrix) or to an empty matrix and incubated for 1 h. After incubation, the resins were extensively washed, and samples of input (Input) or the protein retained by the different matrices were analyzed by SDS-PAGE and exposed for autoradiography.
NS1 and eIF4G interact directly.
Due to the complex behavior of NS1 as an interacting protein, we asked whether NS1-4GI interaction could be detected in vitro using purified proteins and whether the presence of RNA is required. To answer these questions, the region of the eIF4GI protein comprising amino acids 157 to 550 and the full-length NS1 protein were expressed in E. coli as a His-tagged protein and were purified to apparent homogeneity (Fig. 5A, upper left panel). The interaction between the two proteins in native form was studied by using two different approaches. First, we used the overlay technique (30). Various amounts of purified eIF4GI157-550, NS1 protein, or bovine serum albumin as the control were applied to a nitrocellulose filter and were subsequently incubated with 4GI157-550 protein, NS1 protein, or protein A as the negative control (Fig. 5A, bottom panels). The bound protein was fixed with formaldehyde and detected by Western blotting with specific antibodies. The specificity of the antibodies was tested by applying directly the highest amount of either protein (500 ng) to the nitrocellulose filter. Under the conditions used, the antibodies recognized only the specific proteins (Fig. 5A, upper right panel). The results shown in Fig. 5A indicate that both NS1 and eIF4GI157-550 proteins are able to bind each other but not to protein A used as a control. To rule out the possibility that an RNA molecule could mediate the binding between eIF4GI157-550 and NS1, the purified proteins were treated with RNase A prior to the binding assay. No RNA was detectable in the RNase-treated preparations, as assayed by ethidium bromide staining. The results obtained (Fig. 5A) show that interaction of the two proteins is not mediated by an RNA bridge, although the possibility cannot be excluded that RNA fragments protected by NS1 protein could influence the interaction.
Additional binding experiments were carried out by using a matrix containing a GST-NS1 fusion protein or a GST protein as a negative control. Purified His-4GI157-550 protein was loaded onto GST- and GST-NS1-containing matrices, and after incubation and extensive washing as described in Materials and Methods, the retained protein was analyzed in the glutathione eluate by Western assays with anti-His antibodies. The results are presented in Fig. 5B. In contrast to the GST control matrix, the GST-NS1-containing matrix specifically retained a 4GI form that was eluted with glutathione. The 4GI molecule retained presented a lower molecular weight than the bulk of applied protein. The His-4GI157-550 protein contains 393 residues of the amino terminal half of 4GI protein and behaves as a protein with a molecular weight of 125,000. This is in agreement with the abnormal electrophoretic mobility of 4GI protein and, specifically, of its N-terminal sequence (36, 55, 76). The 4GI form retained by the GST-NS1 column presented an apparent molecular weight of around 90,000, and due to the aberrant mobility of this 4GI157-550 protein, it is not possible to establish the actual size of the bound molecule. The decrease in the mobility of the 4GI-bound protein is not due to proteolysis, since the unbound protein after resin incubation still has the original size, in both the control and the NS1-bound resins (Fig. 5B, right panel, unb). Therefore, it is possible that NS1 retains more efficiently a 4GI form of lower molecular weight present in the original preparation or that the 4GI molecule associated with NS1 suffers a conformational change that produces variations in the electrophoretic mobility.
Mapping of the eIF4GI-binding domain in the NS1 protein.
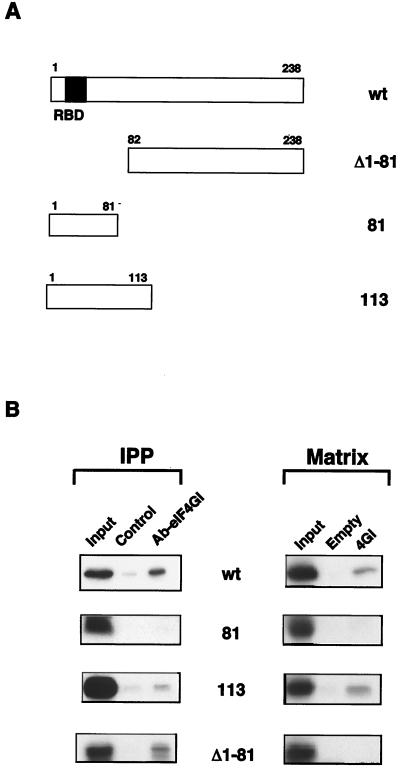
It has been previously reported that a deletion mutant of NS1 protein containing the N-terminal 81 amino acids was not able to enhance the translation of influenza virus messengers, whereas the first 113 amino acids of the protein were totally active for this function (45). Therefore, we studied whether a correlation between enhancement of translation and eIF4GI interaction could be established. To do that, several NS1 deletion mutants were translated in vitro and were used to analyze their ability to bind to eIF4GI protein. For this purpose, two different approaches were carried out, coimmunoprecipitation and pull-down experiments. For coimmunoprecipitation assays with the endogenous eIF4GI, the NS1 protein's translation reactions were adsorbed with protein A-Sepharose, and the supernatants were incubated with a control serum (Fig. 6B, Control) or with an eIF4GI-specific serum (Fig. 6B, Ab-eIF4GI). The immunoprecipitates were washed several times with binding buffer, were boiled in Laemmli sample buffer, and were analyzed by SDS-PAGE (Fig. 6B, IPP). To carry out pull-down experiments, samples of the in vitro translated NS1 proteins were diluted with interaction buffer without imidazole and were preincubated with empty matrix to remove the unspecific binding. The supernatant, containing the labeled NS1 proteins, was applied to a 4GI157-550 matrix or to an empty matrix and washed extensively and the retained protein was analyzed by SDS-PAGE (Fig. 6B, Matrix). The results obtained with both approaches indicated that an NS1 mutant containing the first 113 N-terminal amino acids was able to bind to the initiation factor but a mutant containing the first 81 N-terminal amino acids was not. An NS1 deletion mutant lacking the 81 N-terminal amino acids retained the capacity to interact with eIF4GI, as measured by coimmunoprecipitation analysis (Fig. 6B, Δ1-81). This result confirms that RNA is not required for eIF4GI-NS1 interaction, because this mutant does not have the RNA-binding domain that is located at positions 19 to 38 of the protein (65). For reasons not clear at present, this mutant was not able to bind to a His4GI157-550 matrix. The results presented indicate that a functional correlation exists between the capacity of a mutant NS1 protein to interact with eIF4GI and its ability to stimulate viral translation.
FIG. 6.
Mapping of eIF4GI-binding domain in the NS1 protein. (A) Scheme of the NS1 deletion mutants. (B) Mapping of interacting domains. Total extracts of in vitro-labeled full length NS1 (wt) and deletion mutant proteins were coimmunoprecipitated (IPP) with a control serum (Control) or with a specific anti-eIF4GI serum (Ab-eIF4GI). Alternatively, in vitro translated NS1 proteins were used for pull-down experiments (Matrix) with an empty matrix or with a matrix containing His-4GI157-550 (4GI). Samples of input (Input), of the coimmunoprecipitates, or of the protein retained by the different matrices were analyzed by SDS-PAGE and exposed for autoradiography.
Composition of the eIF4F complex during influenza virus infection.
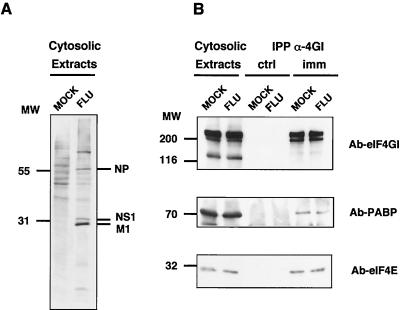
NS1 protein interacts with the N-proximal part of the 4GI component of the eIF4F translation initiation complex, in a region close to the binding sites of eIF4E and PABP proteins. Therefore, it was possible that competition with 4E, PABP, or both with NS1 protein for 4GI interaction took place, and then the influenza virus infection could have affected the amount of 4E and PABP bound to the translational factor. To test the possible competition among these proteins for binding to the 4GI factor, cultures of COS-1 cells were mock infected or infected with influenza virus, and cytoplasmic extracts were prepared at 6 h postinfection, a time at which most newly synthesized proteins are virus specific. The cultures were metabolically labeled with [35S]methionine-cysteine between 3 and 6 h postinfection, and immunoprecipitation analysis was carried out by using specific anti-4GI antibodies. The presence of eIF4E and PABP proteins in the immunoprecipitates was evaluated by Western assays. The results are presented in Fig. 7. The presence of specific labeled proteins from influenza virus-infected cells was clearly visible in the total cell extracts (Fig. 7A). The amounts of eIF4GI, eIF4E, and PABP proteins did not change with the influenza virus infection (Fig. 7B, Cytosolic Extracts), and the amounts of eIF4E and PABP present in the eIF4GI immunoprecipitates were similar in mock-infected and influenza virus-infected cells (Fig. 7B, imm). These results indicate that the total amount of 4E protein or PABP that remains bound to the 4GI component of the eIF4F translation factor does not essentially change with the influenza virus infection, suggesting that the virus is not producing an extensive change in the composition of the eIF4F translation initiation factor.
FIG. 7.
Composition of eIF4F complex in influenza virus-infected cells. Cultures of COS-1 cells were mock infected (MOCK) or infected with influenza virus A/Victoria/3/75 (FLU). The cultures were labeled with [35S]Met-Cys, and cytoplasmic extracts were prepared at 6 h postinfection. (A) Cytosolic extracts were separated in SDS-polyacrylamide gels and exposed for autoradiography. (B) Cytosolic extracts were immunoprecipitated with preimmune (IPP α-4GI, ctrl) or immune (IPP α-4GI, imm) anti-4GI sera. Samples of the immunoprecipitates or the extracts prior to immunoprecipitation (Cytosolic Extracts) were separated in SDS-polyacrylamide gels. The gels were blotted to nitrocellulose and developed in a Western assay using anti-eIF4GI (Ab-eIF4GI), anti-eIF4E (Ab-eIF4E), or anti-PABP (Ab-PABP) serum.
DISCUSSION
eIF4GI, a pivotal element in protein synthesis initiation.
Mammalian eIF4GI has an important role in the initiation of translation. The intact protein is required to mediate cap-dependent translation initiation through mRNA recruitment via the binding of eIF4E to the mRNA cap structure. The C-terminal two-thirds of the protein are sufficient to support cap-independent translation initiation. Translation factor eIF4GI behaves as an adapter molecule, having a highly conserved binding site for the eIF4E protein at the N-terminal part (amino acids 568 to 585) (43). The C-terminal two-thirds contain the binding sites for eIF3 (positions 643 to 1043) (35), which allows the localization of the complex to the ribosome, and the bipartite binding site for the ATP-dependent helicase eIF4A at positions 614 to 877 and 1044 to 1553 (35). An RNA recognition motif has been reported overlapping with the binding site for eIF3 (see reference 49 for a review). Recently, a binding domain for the mitogen-activated protein kinase Mnk1 on the eIF4GI molecule has been described (64). This kinase interacts at the C-terminal end of eIF4GI and could regulate 4E phosphorylation, a step which may modulate 4E cap-binding activity. A new N-terminal extension of the eIF4GI gene has been reported that includes a binding site for PABP (21) (residues 1 to 157; see scheme in Fig. 4A) and that constitutes the actual N terminus of the protein.
The connector nature of eIF4GI has allowed the evolution of different strategies to control protein synthesis using eIF4GI as the target. Thus, a family of proteins has been found, referred to as eIF4E-BPs, that is able to bind eIF4E and compete for its interaction with eIF4GI (39, 43). The interaction of eIF4E with the eIF4E-BPs is regulated by phosphorylation of the latter in a way that is dependent on the physiological state of the cell (8, 25, 44). Phosphorylation of the eIF4E protein increases its cap-binding activity and its capacity to associate with eIF4GI (73), and hence it has been proposed as a mechanism to regulate the initiation of translation.
Virus-cell interactions affecting eIF4GI.
Viruses present a diversity of mechanisms to positively regulate the translation of their messengers, and the study of these mechanisms has provided a more detailed understanding of the protein synthesis machinery. Viruses have also taken advantage of the regulatory potential of eIF4GI-mediated processes to take over cell translation. Hence, picornaviruses lead to the cleavage of eIF4GI protein, generating a C-terminal fragment able to carry out internal initiation. As a consequence of the elimination of the eIF4E-binding site, cellular cap-dependent translation is inhibited. Under these circumstances, translation of the viral messengers that are uncapped but contain an internal ribosome entry site can take place (23, 47). A different strategy is used by rotaviruses: rotavirus mRNAs are capped but not polyadenylated. They contain a 3′ untranslated region of variable length with a strictly conserved sequence. It has been recently reported that the rotavirus protein NSP3A binds specifically such a conserved sequence and the N-terminal region of eIF4GI protein. Binding of the viral protein displaces PABP from the eIF4F complex and leads to the inhibition of cellular translation (60).
During influenza virus infection, the translation machinery remains competent for protein synthesis by eluding the activation of the double-stranded RNA-activated kinase (PKR). Several mechanisms have been proposed as responsible for this PKR regulation: (i) viral induction of a 58-kDa cellular protein that inhibits both PKR autophosphorylation and the phosphorylation of the eukaryotic initiation factor eIF2α (37, 38, 61), (ii) trapping of dsRNA by NS1 (41), and (iii) direct interaction of NS1 with PKR (78) or with hStaufen protein (6). Influenza virus infection causes a switch from cellular to viral protein synthesis, although functional cellular mRNAs are present in the cytoplasm of the infected cells (28). Influenza virus messengers are capped and polyadenylated, but viral mRNAs are preferentially translated at the expense of cellular ones, their 5′ UTR being important for selectivity (13, 14). These observations correlate with the described enhancement of viral translation mediated by NS1 protein (3, 5), also mediated by the 5′ UTR of their mRNAs (3). NS1 is an RNA-binding protein able to recognize poly(A) (66) and the 5′ extracistronic region of viral mRNAs (59), among other RNAs, and able to oligomerize (51).
In this report, we have shown that NS1 protein interacts with eIF4GI in vivo, both in transfected cells and in cells infected with influenza virus (Fig. 1), as well as in vitro (Figs. 2 and 3). Furthermore, the results presented indicate that the interaction is direct, since it also takes place when purified proteins are used in vitro (Fig. 5). Taking into account this interaction, in conjunction with the ability of NS1 protein to bind the 5′ UTR of viral mRNAs, it is conceivable that NS1 may act as a virus-specific initiation factor. NS1 may promote the binding of eIF4F to the 5′ end of viral mRNAs and may compete for the initiation of cellular mRNAs.
Studies in several systems have demonstrated that translational efficiency can be stimulated synergistically when both the cap structure and the poly(A) tail are present (11, 20, 80). These data led to attractive models that propose interaction between the 5′ and 3′ ends of the mRNA molecule in a closed-loop structure that would bring about an efficient reinitiation (10, 24, 72, 73). The interaction between eIF4GI and NS1, together with the RNA-binding activity of NS1 toward the viral mRNA 5′ end and the poly(A) tract and its oligomerization ability, could induce the circularization of the influenza virus messengers, enhancing their translation by shunting terminating ribosomes directly to the 5′ end of the mRNAs.
Relevance of NS1-eIF4GI interaction for influenza virus infection.
As indicated above, expression of NS1 protein induces alterations at several posttranscriptional steps in cellular gene expression (56). These effects have been correlated with the interaction of NS1 protein with cellular RNA or protein targets. Thus, the interaction of NS1 protein with the following has been reported: a protein related to estradiol 17β-dehydrogenase (81), the 30-kDa subunit of the cleavage and polyadenylation specificity factor (50), the poly(A)-binding protein II (2), NS1-BP, a human 70-kDa protein localized in nuclear regions enriched with the spliceosome assembly factor SC35 (82), the human homologue of Staufen protein (hStaufen) (6, 46), and eIF4GI (this report).
The phenotypes of mutant influenza viruses affected in the NS1 gene reflect the diversity of activities that could be assigned to this protein: some temperature-sensitive mutants are affected in viral gene expression at a posttranscriptional level (17), while another is defective in viral transcription (29). On the other hand, more profound alterations in the NS1 gene, recently generated by reverse genetics, indicate that NS1 expression is not absolutely essential for virus multiplication in cell culture. Thus, large C-terminal deletions of the protein lead to viruses with reduced replication capacity in normal tissue culture cells but with a normal phenotype in a cell line defective in interferon genes (4). Even a virus lacking the NS1 gene was able to replicate efficiently under conditions of no interferon response but was severely affected in normal cells (12). This genetic evidence, together with the results obtained by expression of NS1 cDNA and the interactions of the protein with several cell factors, indicates that this small protein is a key element in the interplay of the virus with the cell posttranscriptional processes, whose function relates to the optimal utilization of cell resources for virus multiplication.
ACKNOWLEDGMENTS
We are indebted to Thomas Zürcher, Agustín Portela and José A. Melero for their critical comments on the manuscript. We thank R. E. Rhoads and N. Sonenberg for providing biological materials. The technical assistance of Y. Fernández and J. Fernández is gratefully acknowledged.
T. Aragón was a fellow from Gobierno Vasco. This work was supported by Programa Sectorial de Promoción General del Conocimiento (grants PM-0015, PB94-1542, and PB97-1160).
REFERENCES
- 1.Beloso A, Martínez C, Valcárcel J, Fernández-Santarén J, Ortín J. Degradation of cellular mRNA during influenza virus infection: its possible role in protein synthesis shutoff. J Gen Virol. 1992;73:575–581. doi: 10.1099/0022-1317-73-3-575. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Li Y, Krug R M. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Luna S, Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J Virol. 1995;69:2427–2433. doi: 10.1128/jvi.69.4.2427-2433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egorov A, Brandt S, Sereining S, Romanova J, Ferko B, Katinger D, Grassauer A, Alexandrova G, Katinger H, Muster T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J Virol. 1998;72:6437–6441. doi: 10.1128/jvi.72.8.6437-6441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enami K, Sato T A, Nakada S, Enami M. Influenza virus NS1 protein stimulates translation of the M1 protein. J Virol. 1994;68:1432–1437. doi: 10.1128/jvi.68.3.1432-1437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falcón A, Fortes P, Marión R M, Beloso A, Ortín J. Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro. Nucleic Acids Res. 1999;27:2241–2247. doi: 10.1093/nar/27.11.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigenblum D, Schneider R J. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J Virol. 1993;67:3027–3035. doi: 10.1128/jvi.67.6.3027-3035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn A, Proud C G. Ser209, but not Ser35, is the major site of phosphorylation in initiation factor eIF4E in serum treated Chinese hamster ovary cells. J Biol Chem. 1995;270:21684–21688. doi: 10.1074/jbc.270.37.21684. [DOI] [PubMed] [Google Scholar]
- 9.Fortes P, Beloso A, Ortín J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks RNA nucleocytoplasmic transport. EMBO J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallie D R. Translational control of cellular and viral mRNAs. Plant Mol Biol. 1996;32:145–158. doi: 10.1007/BF00039381. [DOI] [PubMed] [Google Scholar]
- 11.Gallie D R, Tanguay R. Poly(A) binds to initiation factors and increases cap-dependent translation in vitro. J Biol Chem. 1994;269:17166–17173. [PubMed] [Google Scholar]
- 12.García-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 13.Garfinkel M S, Katze M G. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAS in infected cells. J Biol Chem. 1992;267:9383–9390. [PubMed] [Google Scholar]
- 14.Garfinkel M S, Katze M G. Translational control by influenza virus. Selective translation is mediated by sequences within the viral mRNA 5′-untranslated region. J Biol Chem. 1993;268:22223–22226. [PubMed] [Google Scholar]
- 15.Gluzman Y. SV40 transformed simian cells support the replication or early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 16.Hatada E, Fukuda R. Binding of influenza A virus NS1 protein to dsRNA in vitro. J Gen Virol. 1992;73:3325–3329. doi: 10.1099/0022-1317-73-12-3325. [DOI] [PubMed] [Google Scholar]
- 17.Hatada E, Hasegawa M, Shimizu K, Hatanaka M, Fukuda R. Analysis of influenza A virus temperature-sensitive mutants with mutations in RNA segment 8. J Gen Virol. 1990;71:1283–1292. doi: 10.1099/0022-1317-71-6-1283. [DOI] [PubMed] [Google Scholar]
- 18.Hatada E, Saito S, Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatada E, Saito S, Okishio N, Fukuda R. Binding of the influenza virus NS1 protein to model genome RNAs. J Gen Virol. 1997;78:1059–1063. doi: 10.1099/0022-1317-78-5-1059. [DOI] [PubMed] [Google Scholar]
- 20.Iizuka N, Najita L, Franzusoff A, Sarnow P. Cap-dependent and cap-independent translation by internal initiation of mRNAs in cell extracts prepared from Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:7322–7330. doi: 10.1128/mcb.14.11.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imataka H, Gradi A, Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inglis S C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982;2:1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson R J, Kaminski A. Internal initiation of translation in eukaryotes: the picornaviral paradigm and beyond. RNA. 1995;1:985–1000. [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson A. Poly(A) metabolism and translation: the closed loop model. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 451–480. [Google Scholar]
- 25.Joshi B. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at ser209. J Biol Chem. 1995;270:14597–14603. doi: 10.1074/jbc.270.24.14597. [DOI] [PubMed] [Google Scholar]
- 26.Joshi B, Yan R, Rhoads R E. In vitro synthesis of human protein synthesis initiation factor 4γ and its localization on 43 and 48 S initiation complexes. J Biol Chem. 1994;269:2048–2055. [PubMed] [Google Scholar]
- 27.Katze M G, DeCorato D, Krug R M. Cellular mRNA translation is blocked at both initiation and elongation after infection by influenza virus or adenovirus. J Virol. 1986;60:1027–1039. doi: 10.1128/jvi.60.3.1027-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katze M G, Krug R M. Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol Cell Biol. 1984;4:2198–2206. doi: 10.1128/mcb.4.10.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koennecke I, Boschek C B, Scholtissek C. Isolation and properties of a temperature-sensitive mutant (ts412) of the influenza virus recombinant with a lesion in the gene coding for the nonstructural protein. Virology. 1981;110:16–25. doi: 10.1016/0042-6822(81)90003-9. [DOI] [PubMed] [Google Scholar]
- 30.Kremer L, Domínguez J, Avila J. Detection of tubulin-binding proteins by an overlay-assay. Anal Biochem. 1988;175:91–95. doi: 10.1016/0003-2697(88)90365-x. [DOI] [PubMed] [Google Scholar]
- 31.Krug R M, Alonso-Kaplen F V, Julkunen I, Katze M G. Expression and replication of the influenza virus genome. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum Press; 1989. pp. 89–152. [Google Scholar]
- 32.Krug R M, Etkind P R. Cytoplasmic and nuclear specific proteins in influenza virus-infected MDCK cells. Virology. 1973;56:334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- 33.Lamb R A. The genes and proteins of influenza viruses. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum Press; 1989. pp. 1–87. [Google Scholar]
- 34.Lamb R A, Choppin P W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci USA. 1979;76:4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamphear B J, Kirchweger R, Skern T, Rhoads R E. Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornavirus proteases: implication for cap-dependent and cap-independent translational initiation. J Biol Chem. 1995;270:21975–21983. doi: 10.1074/jbc.270.37.21975. [DOI] [PubMed] [Google Scholar]
- 36.Lamphear B J, Yan R, Yang F, Waters D, Liebig H D, Klump H, Kuechler E, Skern T, Rhoads R E. Mapping the cleavage site in protein synthesis initiation factor eIF-4gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993;268:19200–19203. [PubMed] [Google Scholar]
- 37.Lee T G, Tomita J, Hovanessian A G, Katze M G. Purification and partial characterization of a cellular inhibitor of the interferon-induced protein kinase of Mr 68,000 from influenza virus-infected cells. Proc Natl Acad Sci USA. 1990;87:6208–6212. doi: 10.1073/pnas.87.16.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee T G, Tomita J, Hovanessian A G, Katze M G. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J Biol Chem. 1992;267:14238–14243. [PubMed] [Google Scholar]
- 39.Lin T A, Kong X, Haystead T A J, Pause A, Belsham G J, Sonenberg N, Lawrence J C J. PHAS-1 as a link between mitogen-activated protein kinase and translation initiation. Science. 1994;266:653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Qian X Y, Krug R M. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 1994;8:1817–1828. doi: 10.1101/gad.8.15.1817. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Wambach M, Katze M G, Krug R M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the e1F-2 translation initiation factor. Virology. 1995;214:222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 42.Luo G X, Luytjes W, Enami M, Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991;65:2861–2867. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-γ and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makkinje A, Xiong H, Li M, Damuni Z. Phosphorylation of eukaryotic protein synthesis initiation factor 4E by insulin-stimulated protamine kinase. J Biol Chem. 1995;270:14824–14838. doi: 10.1074/jbc.270.24.14824. [DOI] [PubMed] [Google Scholar]
- 45.Marión R M, Aragón T, Beloso A, Nieto A, Ortín J. The N-terminal half of the influenza virus NS1 protein is sufficient for nuclear retention of mRNA and enhancement of viral mRNA translation. Nucleic Acids Res. 1997;25:4271–4277. doi: 10.1093/nar/25.21.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marión R M, Fortes P, Beloso A, Dotti C, Ortín J. A human sequence homologue of Staufen is an RNA binding protein that is associated to polysomes and localizes to the rough endoplasmic reticulum. Mol Cell Biol. 1999;19:2212–2219. doi: 10.1128/mcb.19.3.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meerovitch K, Sonenberg N. Internal initiation of picornavirus RNA translation. Semin Virol. 1993;4:217–227. [Google Scholar]
- 48.Merrick W C, Hershey J W B. The pathway and mechanism of protein synthesis. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–70. [Google Scholar]
- 49.Morley S J, Curtis P S, Pain V M. eIF4G: translation's mystery factor begins to yield its secrets. RNA. 1997;3:1085–1104. [PMC free article] [PubMed] [Google Scholar]
- 50.Nemeroff M E, Barabino S M L, Li Y, Keller W, Krug R M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 51.Nemeroff M E, Qian X Y, Krug R M. The influenza virus NS1 protein forms multimers in vitro and in vivo. Virology. 1995;212:422–428. doi: 10.1006/viro.1995.1499. [DOI] [PubMed] [Google Scholar]
- 52.Nieto A, de la Luna S, Bárcena J, Portela A, Valcárcel J, Melero J A, Ortín J. Nuclear transport of influenza virus polymerase PA protein. Virus Res. 1992;24:65–75. doi: 10.1016/0168-1702(92)90031-4. [DOI] [PubMed] [Google Scholar]
- 53.Novoa I. Ph.D. thesis. Madrid, Spain: Universidad Autonoma; 1996. [Google Scholar]
- 54.Novoa I, Cotten M, Carrasco L. Hybrid proteins between Pseudomonas aeruginosa exotoxin A and poliovirus 2Apro cleave p220 in HeLa cells. J Virol. 1996;70:3319–3324. doi: 10.1128/jvi.70.5.3319-3324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novoa I, Martinez-Abarca F, Fortes P, Ortín J, Carrasco L. Cleavage of p220 by purified poliovirus 2A(pro) in cell-free systems: effects on translation of capped and uncapped mRNAs. Biochemistry. 1997;36:7802–7809. doi: 10.1021/bi9631172. [DOI] [PubMed] [Google Scholar]
- 56.Ortín J. Multiple levels of posttranscriptional regulation of influenza virus gene expression. Semin Virol. 1998;8:335–342. [Google Scholar]
- 57.Ortín J, Nájera R, López C, Dávila M, Domingo E. Genetic variability of Hong Kong (H3N2) influenza viruses: spontaneous mutations and their location in the viral genome. Gene. 1980;11:319–331. doi: 10.1016/0378-1119(80)90072-4. [DOI] [PubMed] [Google Scholar]
- 58.Pain V M. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 59.Park Y W, Katze M G. Translational control by influenza virus. Identification of cis-acting sequences and trans-acting factors which may regulate selective viral mRNA translation. J Biol Chem. 1995;270:28433–28439. doi: 10.1074/jbc.270.47.28433. [DOI] [PubMed] [Google Scholar]
- 60.Piron M, Vende P, Cohen J, Poncet D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998;17:5811–5821. doi: 10.1093/emboj/17.19.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polyak S J, Tang N, Wambach M, Barber G N, Katze M G. The P58 cellular inhibitor complexes with the interferon-induced, double-stranded RNA-dependent protein kinase, PKR, to regulate its autophosphorylation and activity. J Biol Chem. 1996;271:1702–1707. doi: 10.1074/jbc.271.3.1702. [DOI] [PubMed] [Google Scholar]
- 62.Poon L L M, Pritlove D C, Sharps J, Brownlee G G. The RNA polymerase of influenza virus, bound to the 5′ end of virion RNA, acts in cis to polyadenylate mRNA. J Virol. 1998;72:8214–8219. doi: 10.1128/jvi.72.10.8214-8219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pritlove D C, Poon L L, Fodor E, Sharps J, Brownlee G G. Polyadenylation of influenza virus mRNA transcribed in vitro from model virion RNA templates: requirements for 5′ conserved sequences. J Virol. 1998;72:1280–1286. doi: 10.1128/jvi.72.2.1280-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pyronnet S, Imataka H, Gingras A-C, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits Mnk 1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian X-Y, Alonso-Caplen F, Krug R M. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J Virol. 1994;68:2433–2441. doi: 10.1128/jvi.68.4.2433-2441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu Y, Krug R M. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A) J Virol. 1994;68:2425–2432. doi: 10.1128/jvi.68.4.2425-2432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu Y, Nemeroff M, Krug R M. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA. 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 68.Rhoads R E. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993;268:3017–3020. [PubMed] [Google Scholar]
- 69.Richter-Cook N J, Devert T E, Hensold J O, Merrick W C. Purification and characterization of a new eukaryotic protein translation factor. J Biol Chem. 1998;273:7579–7587. doi: 10.1074/jbc.273.13.7579. [DOI] [PubMed] [Google Scholar]
- 70.Robertson J S, Schubert M, Lazzarini R A. Polyadenylation sites for influenza mRNA. J Virol. 1981;38:157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose J K, Buonocore L, Whitt M A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. BioTechniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 72.Sachs A B, Buratowski S. Common themes in translation and transcriptional regulation. Trends Biochem. 1997;22:189–192. doi: 10.1016/s0968-0004(97)01051-7. [DOI] [PubMed] [Google Scholar]
- 73.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 74.Sanz-Ezquerro J J, de la Luna S, Ortín J, Nieto A. Individual expression of influenza virus PA protein induces degradation of coexpressed proteins. J Virol. 1995;69:2420–2426. doi: 10.1128/jvi.69.4.2420-2426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skehel J J. Polypeptide synthesis in influenza-virus infected cells. Virology. 1972;49:23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- 76.Sommergruber W, Ahorn H, Klump H, Seipelt J, Zoephel A, Fessl F, Krystek E, Blaas D, Kuechler E, Liebig H, Skern T. 2A proteinases of coxsackie- and rhinovirus cleave peptides derived from eIF-4 gamma via a common recognition motif. Virology. 1994;198:741–745. doi: 10.1006/viro.1994.1089. [DOI] [PubMed] [Google Scholar]
- 77.Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 78.Tan S L, Katze M G. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J Interferon Cytokine Res. 1998;9:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 79.Tarun S Z, Sachs A B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 80.Tarun S Z J, Sachs A B. A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 1995;9:2997–3007. doi: 10.1101/gad.9.23.2997. [DOI] [PubMed] [Google Scholar]
- 81.Wolff T, O'Neill R E, Palese P. Interaction cloning of NS1-I, a human protein that binds to the nonstructural NS1 proteins of influenza A and B viruses. J Virol. 1996;70:5363–5372. doi: 10.1128/jvi.70.8.5363-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolff T, O'Neill R E, Palese P. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J Virol. 1998;72:7170–7180. doi: 10.1128/jvi.72.9.7170-7180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]