Angiostrongylus cantonensis Nematode Invasion Pathway, Mallorca, Spain (original) (raw)
Abstract
Neural angiostrongyliasis is an emerging zoonosis caused by the rat lungworm, Angiostrongylus cantonensis. In humans, infection with this nematode often results in eosinophilic meningitis and other severe disorders of the central nervous system. Europe was deemed a nonendemic region until 2018, when A. cantonensis worms were detected on the Mediterranean island of Mallorca, Spain, a tourism hotspot. Since that time, a sentinel surveillance system and a molecular approach have been used to follow the invasion path of the rat lungworm on the island. A. cantonensis worms have been found in animals from 8 locations on the island over 3 consecutive years. Our preliminary results show a recognizable pattern of clinical signs in infected hedgehogs and a single mitochondrial haplotype circulating in Mallorca. We present strong evidence confirming that the rat lungworm has successfully established and colonized an island in Europe and discuss observations and possible strategies for its early detection across continental Europe.
Keywords: Angiostrongylus cantonensis, infection, angiostrongyliasis, human eosinophilic meningitis, meningitis/encephalitis, rat lungworm infection, helminths, parasites, sentinel species, animal diseases, central nervous system, parasitic infections, nematodes, invasion pathway, vector-borne infections, hedgehogs, zoonoses, Mallorca, Spain
The rat lungworm, Angiostrongylus cantonensis, infects animals and humans. Although this nematode species is recognized as the main etiologic agent of eosinophilic meningitis (1), infection might result in other central nervous system disorders (2). Clinical manifestations are aggravated by movement and subsequent death of the worms in the central nervous system, causing physical lesions and inflammation in accidental hosts (3). In humans, severe headache, neck stiffness, paresthesia, convulsions, urinary failure, visual impairment, and other symptoms, occasionally leading to coma and death, have been reported (1,4).
The life cycle of A. cantonensis worms includes rats as definitive and gastropods as intermediate hosts; crustaceans, planarians, amphibians, reptiles, and fish might act as paratenic hosts (2). More than 20 vertebrate species, including humans, have been reported as A. cantonensis lungworm accidental hosts (5). This long list of vertebrate hosts includes nonhuman primates (6), marsupials (7), bats (8), horses (9), dogs (10), birds (11), and more recently, hedgehogs (12). The role of hedgehogs in the transmission of this parasite remains to be clarified.
A. cantonensis worms were detected in Canton, China, infecting the lungs of rats (13) and a decade later, in the cerebrospinal fluid of a person from Taiwan (14). For decades, disease-endemic areas were limited to the Pacific basin and Southeast Asia, but this parasite has spread to new territories at an alarming rate (1). The invasion of A. cantonensis lungworms has been associated with unintended importation of infected rats and gastropods on ships (2,15). Almost 3,000 cases of human neuroangiostrongyliasis have been reported (16) from 30 territories (3), although the prevalence might be higher (17).
Europe was considered to be nonendemic for A. cantonensis worms until 2018 when the parasite was reported infecting the brains of 2 hedgehogs on the Mediterranean island of Mallorca (12). Although the rat lungworm had been previously reported on Tenerife, a subtropical, non-European overseas oceanic island (18), its detection in Mallorca is an indisputable indication of its presence in Europe (5). Mallorca is a major Mediterranean tourism hotspot, highly interconnected with continental Europe. After the detection, the question remained whether A. cantonensis nematodes could survive the temperate winters of Europe. The purpose of this study was to use sentinel surveillance for symptomatic fauna to confirm whether the rat lungworm has been successfully established on Mallorca.
Methods
Surveillance Strategy
We conducted sentinel surveillance of hedgehogs that had signs of disease during 2018‒2020 for early detection of A. cantonensis lungworm‒positive animals on Mallorca. Availability of animals was contingent on local citizens providing injured, ill, or orphaned North African hedgehogs (Atelerix algirus) to the Consorci per a la Recuperació de la Fauna de les Illes Balears wildlife hospital. Animals showing neurologic clinical signs were hospitalized, and their behavior was observed daily.
When possible, a blood sample was obtained from the animal’s jugular vein and sent to an external laboratory (Laboratorio Echevarne S.A., https://laboratorioechevarne.com) for hematologic and clinical chemistry analyses. Blood extraction was not always possible in severely ill or dehydrated hedgehogs. We euthanized critically ill animals to avoid suffering and then subjected them to necropsy, performed in a BioSafety Level 2 facility, according to the regulations of the University of the Balearic Islands. We kept lungs, heart, and head frozen for further analysis.
Detection and Morphologic Identification
We opened preserved skulls by using a scalpel and making 2 parallel incisions along the frontal and parietal bones to access the brain underneath. We completely removed the brain and macroscopically examined the interior of the skull and the subarachnoid space of the brain by using a stereomicroscope (magnification ×10–40). We conducted external examination of the lungs, heart and pulmonary arteries according to the same procedure. We collected nematodes from the brain and the skull’s inner surface.
During 2018, we detected parasites macroscopically, During 2019 and 2020, we changed the method approach and used a tissue digestion technique after the visual inspection. When worms were present, we tentatively identified them as A. cantonenisis nematodes by their typical barber’s pole appearance, which results from spiral disposition of the blood-filled intestine and the white uterine tubes in fully developed female worms. This characteristic can be observed in other Angiostrongylus species. Using the morphologic keys of Chen (13) and Kinsella (19), we also identified male nematodes on the basis of characteristics of the copulatory bursa, with a small dorsal ray, shorter than the externodorsal ones, and by the presence of long spicules (1–1.4 μm). We identified female worms on the basis of the form of their ventrally curved posterior end. We distinguished adults from larvae by their body size and development of the sexual apparatus.
Molecular Identification and Phylogenetic Analysis
We conducted molecular analysis to confirm the morphologic identifications. We extracted genomic DNA by using an NZY Tissue gDNA Isolation Kit (Nzytech, https://www.nzytech.com) and amplified a fragment of the cytochrome c oxidase subunit I (COI) gene region by PCR using primers COI forward, 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′, and COI reverse, 5′-TAAAGAAAGAACATAATGAAAATG-3′ (20). The 50-μL PCR contained 2 μL of genomic DNA, 2 μL of each primer (10 mmol/L), 2 μL of 50 mmol/L MgCl2, 25 μL of Taq Master Mix (Supreme NZY_Taq_II 2x Green Master Mix; Nzytech), and 17 μL of water. We performed PCRs in a Verity Thermo Cycler (Applied Biosystems, https://www.thermofisher.com) as follows: 1 cycle of initial denaturation at 95°C for 3 min; followed by 35 cycles at 95°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 10 min.
We visualized PCR products by electrophoresis on a 2% agarose gel containing Pronasafe Nucleic Acid Stain (Conda Laboratories, https://www.condalab.com). We purified samples by using an NZYGelpure Purification Kit (Nzytech) according to manufacturer specifications. We performed Sanger sequencing by Sistemas Genómicos S.L. (https://www.sistemasgenomicos.com). One A. cantonensis specimen/infected hedgehog was sequenced.
We conducted BLAST analysis (https://blast.ncbi.nlm.nih.gov) of the resulting sequences and used the GenBank database to confirm the identification of the parasites. We retrieved the top 78 hits corresponding with COI sequences of A. cantonensis nematodes for further phylogenetic analysis. We aligned retrieved sequences from GenBank and those obtained in this study by using CodonCode Aligner version 9.0.1 (CodonCode Co., https://www.codoncode.com). We inferred a maximum-likelihood phylogenetic tree by using MEGAX software (https://www.megasoftware.net) with Kimura 2-parameter and 500 bootstrap replicates.
Results
In a 3-year period, 8 animals that had signs of disease were rescued by local citizens from different parts of Mallorca. These animals had clinical signs compatible with a neurologic disease: astasia, pelvic limb ataxia, atonia, asthenia, paresis, and behavioral decay. Five of these animals were females (3 adults, 2 juveniles) and 3 were males (2 adults, 1 juvenile). The age of the hedgehogs was calculated according to Garcia-Salguero et al. (21). The first 2 hedgehogs received were reported previously (12). The common clinical signs in infected hedgehogs were astasia, defined as the inability to stand and walk; lateral recumbency (present in all examined hedgehogs), defined as lying on their side; and bicycling movement (present in 6/8 hedgehogs), defined as a consistent, synchronized movement of the limbs (Video). Bicycling often resulted in skin lacerations.
Video.
Recognizable pattern of clinical signs in hedgehog infected with Angiostrongylus cantonensis lungworm, Mallorca, Spain, showing typical clinical manifestations: astasia, lateral recumbency, and bicycling movement of the limbs.
Infected hedgehogs were found in 8 localities from 7 of municipalities in Mallorca (Table; Figure 1). These locations varied from typical coastal places (hedgehogs AaAL1 and AaAN1) (the abbreviation Aa indicates the name of the hedgehog species [A. algirus]), in which tourism is the most prominent economic activity, to traditional inland rural areas dedicated to farmland (hedgehogs AaSP1 and AaSM1) (Figure 1). With the exception of hedgehog AaSN1, all specimens were found in municipalities located at the foot of the eastern foothills of the Tramuntana Mountain range. Two hedgehogs showed positive results during 2018 and 2019, and 4 hedgehogs showed positive results during 2020. All positive hedgehogs harbored A. cantonensis adults. None of the female worms had eggs.
Table. Details and clinical information for Angiostrongylus cantonensis lungworm‒infected hedgehogs hospitalized at the Consorci per a la Recuperació de la Fauna de les Illes Balears wildlife hospital, Mallorca, Spain.
| Hedgehog specimen | Location | Date | Clinical manifestations | No. lungworms recovered | Helminth co-infections | |
|---|---|---|---|---|---|---|
| On skull | In brain | |||||
| AaAN1* | Camp de Mar (Andratx) | 2018 Oct 13 | Pelvic limb ataxia, atonia, asthenia, behavioral decay, lateral recumbency | 0 | 1 | None |
| AaPA1* | Son Castelló (Palma) | 2018 Oct 23 | Pelvic limb ataxia, atonia, behavioral decay, lateral recumbency | 0 | 5 | None |
| AaSP1 | Sa Pobla | 2019 Nov 11 | Asthenia, astasia, bicycling movements, lateral recumbency, skin lacerations | 1 male | 0 | Crenosoma striatum (lungs) |
| AaAL1 | Alcúdia | 2019 Dec 23 | Astasia, bicycling movements, lateral recumbency | 0 | 2 female, 2 male, 4 damaged specimens | Crenosoma striatum (lungs) |
| AaSM1 | Santa Maria del Camí | 2020 Jan 28 | Astasia, bicycling movements, lateral recumbency | 0 | 11 female, 191 male, 7 damaged specimens | None |
| AaIN1 | Inca | 2020 Oct 28 | Astasia, bicycling movements, skin lacerations, lateral recumbency | 0 | 6 female, 3 male, 9 damaged specimens | Crenosoma striatum (lungs) |
| AaPA2 | Establiments (Palma) | 2020 Nov 26 | Astasia, bicycling movements, lateral recumbency | 2 female, 1 male, 1 damaged specimen | 33 female, 20 male, 11 damaged specimens | Crenosoma striatum (lungs) |
| AaSN1 | Calonge (Santanyí) | 2020 Dec 28 | Astasia, repetitive cycling movements, lateral recumbency | 0 | 2 female | Crenosoma striatum (lungs) |
Figure 1.
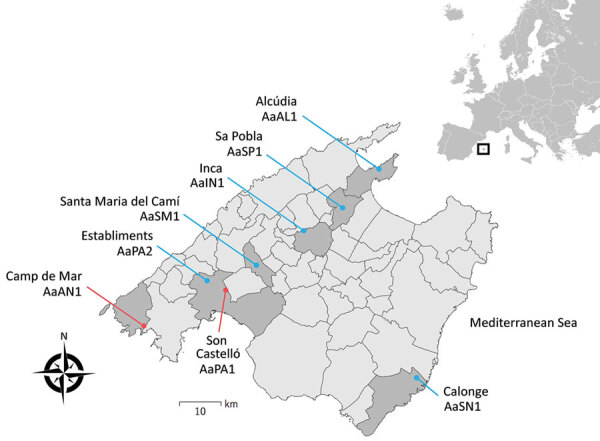
Geographic location of hedgehogs infected by Angiostrongylus cantonensis rat lungworms, Mallorca, Spain, 2018–2020. Blue indicates new cases reported in this study (see Table for details), and red indicates cases reported previously (12). Inset shows location of Mallorca off the coast of southwestern Europe.
Infected hedgehogs were found during autumn to early winter, specifically during September, October, and December (Table). None of the A. cantonensis specimens were found in the lungs or hearts of infected hedgehogs. All but 2 positive hedgehogs were co-infected with the lungworm Crenosoma striatum. Hematologic analysis could only be conducted for 2 infected hedgehogs. Hedgehog AaSP1 had a blood eosinophil count of 4% and an absolute blood count of 0.836 × 103 cells/μL, and hedgehog AaAL1 had a blood eosinophil count of 2% and an absolute blood count of 0.208 × 103 cells/μL.
DNA Assessment
After ClustalW alignment (https://www.ebi.ac.uk), we obtained a 389-bp sequence of the COI gene region. DNA extraction was not successful for parasites from hedgehog AaAL1. All remaining DNA sequences resulted in the same CI haplotype, the same one that was reported by our group in 2019 (12). We subjected the haplotype sequence to BLAST analysis against the GenBank database. The top 78 hits corresponded with COI sequences of A. cantonensis nematodes; the first 5 sequences showed 100% identity. Maximum-likelihood analysis resulted in a phylogenetic tree lacking strong bootstrap support values at deeper nodes (Figure 2). Specimens from Mallorca were clustered in the same clade as those from Tenerife (Canary Islands, Spain), Australia, Taiwan, and New Orleans, Louisiana, USA.
Figure 2.
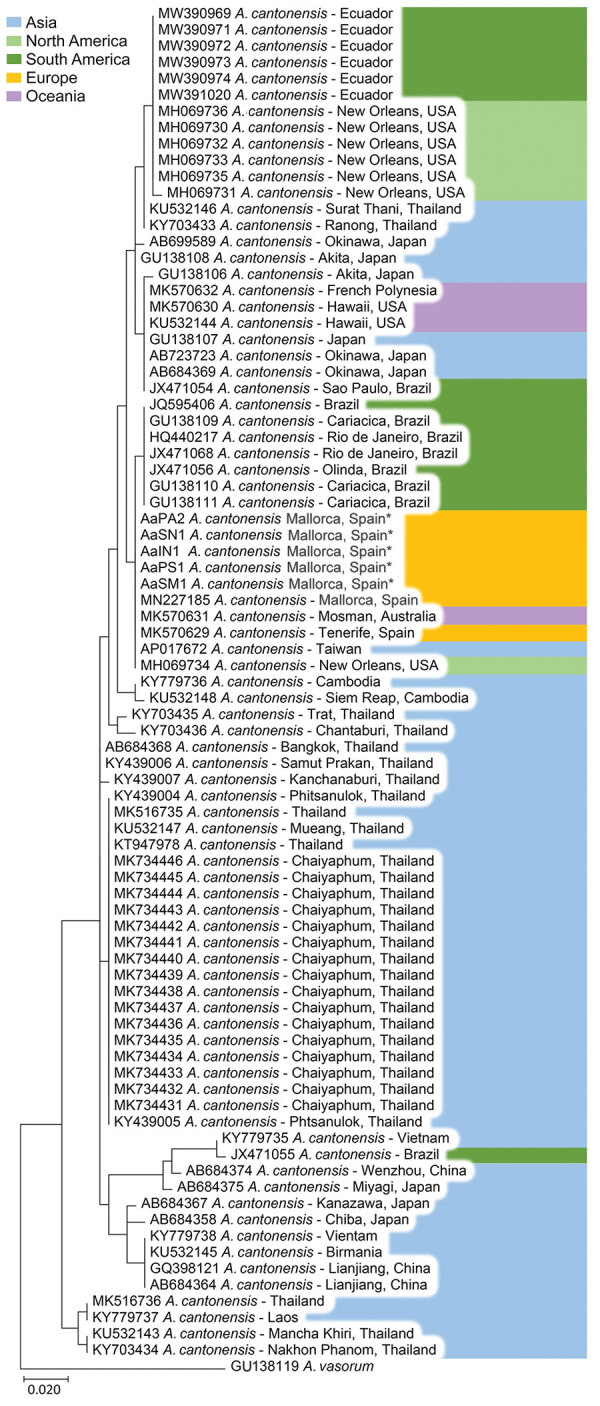
Maximum-likelihood tree showing the phylogenetic position of Angiostrongylus cantonensis rat lungworm cytochrome c oxidase subunit I gene fragments generated in study of infected hedgehogs in Mallorca, Spain, 2018–2020 (asterisks), and reference sequences retrieved from GenBank (accession numbers shown).
Discussion
This study showed that the invasive neurotropic parasite A. cantonensis, the rat lungworm, is the main cause of neurologic disease in North African hedgehogs on the Mediterranean island of Mallorca. The rat lungworm has been found in hedgehogs from 8 locations in Mallorca over 3 consecutive years, indicating that this parasite is spreading and has successfully established in this territory of Europe since 2018.
Sentinel surveillance of hedgehogs that had signs of disease has resulted in a powerful and inexpensive public health monitoring tool to follow invasion of A. cantonensis lungworms in Mallorca. Hedgehogs are ubiquitous in Europe, and they have been reported as the most common mammal admitted to wildlife hospitals in Europe, where their clinical signs can be monitored closely (21,22). Despite the proven utility of this strategy, sentinel surveillance is often underused for detecting emerging pathogens (23).
Other mammals have been proposed as sentinels for early detection and understanding of the dynamics of A. cantonensis transmission: for example, the tawny frogmouth Podargus strigoides in Australia (24) because of its abundance and ubiquity (25), and dogs because of their clear clinical manifestations (26). We found a high (100%) prevalence of A. cantonensis worms in animals showing neurologic signs. In positive hedgehogs, the most predictive signs were astasia, lateral recumbency, and bicycling movement. These clinical manifestations might be used for presumptive diagnosis of an A. cantonensis infection in wildlife hospitals in Europe. More studies are necessary to validate these observations.
Characteristic neurologic signs of A. cantonensis infection have also been observed in tawny frogmouths. Ma et al. detected the parasite in 80% of symptomatic birds, in which paresis/paralysis affecting the hind limbs was the most common clinical manifestation (25). Progressive ascending paralysis of the limbs has also been observed in dogs (27). The gastropod-borne nematode C. striatum was present in most rat lungworm‒positive hedgehogs in our study. This finding is not surprising because the prevalence of this lungworm in Mallorca is high (S. Delgado-Serra, unpub. data) but indicates that both parasites can co-infect the lungs of these mammals. Conversely, eosinophil count was unremarkable. The absence of eosinophilia in peripheral blood has also been observed in other animals (28) and humans (29) positive for this infection.
We found preliminary evidence of an apparent seasonality of neural angiostrongyliasis in Mallorca; all cases were detected in autumn and early winter (October‒December). This seasonal pattern has also been observed in dogs in eastern Australia (26). However, cases in tawny frogmouths, also in eastern Australia, occur in late summer and autumn (25). Instead of seasonality, prevalence of neural angiostrongyliasis might reflect periods of increased precipitation because this increase has a direct effect on the availability of snails and slugs (30).
Mallorca is an endemic foci of the rat lungworm in Europe; however, intermediate hosts in this region remain to be determined. To date, Egypt and Mallorca are the only rat lungworm–endemic territories in the Mediterranean Basin. Although none of the intermediate hosts reported in Egypt are present in Mallorca, the snail species Theba pisana and Cornu aspersum, reported in the Canary Islands (31) are also present in Mallorca. Both species are widely distributed in continental Europe.
All lungworms we sequenced had the same haplotype and were 100% congruent with those reported in Australia, New Orleans, Taiwan, and Tenerife. The single haplotype found in all specimens might be explained by recent range expansion of this parasite and might be the result of a single colonization event. However, more studies are needed to investigate the invasion origin of this parasite species.
Some open questions and limitations of this study should be discussed. First, we cannot know the exact locations where the parasite is circulating in Mallorca because the extent of the home range of North African hedgehogs can be >90 hectares/day (32). Surveillance should then include rats, which have smaller home ranges (33), or gastropods, especially because these hosts are far more abundant and widespread than hedgehogs. Second, the data presented do not reflect the real status of neural angiostrongyliasis in hedgehogs in Mallorca because we have only examined animals rescued by citizens. Third, the role of hedgehogs within the living cycle of the parasite is unknown. In 2018, our group found a gravid A. cantonensis female worm in the brain of a hedgehog (12), indicating that the parasite might reach sexual maturity in this host. However, we found no gravid female subsequently. Whether hedgehogs act as definitive hosts requires further research.
The heavy traffic of ships between the Balearic Islands and continental Europe might have already resulted in the introduction of infected rats to the mainland, and A. cantonensis lungworms might be more widely distributed on the continent than previously believed (5). Furthermore, the Mediterranean region confronts its own challenges in relation to the arrival of the rat lungworm. Snails are a major part of the Mediterranean diet, which has resulted in an increase of snail farms in the region (34). Food safety agencies on the continent might be aware of the increasing challenges for this industry because of possible introduction of the rat lungworm. The first detections of these worms in nonendemic areas often occur after the report of fatal human cases (35–37). In other regions, infections in wild, domestic, and captive animals have preceded those in humans (28), providing the ideal sequence of events to raise early public awareness and to establish early prevention strategies. A delay in the diagnosis and treatment for patients often results in worse prognosis. We recommend adopting a sentinel surveillance and One Health approaches similar to the one we provide in this study for the early detection of the rat lungworm in wildlife hospitals across Europe. However, this strategy should not replace the traditional means of detecting the rat lungworm. Further efforts should include increasing public and medical awareness of neuroangiostrongyliasis and conducting systematic surveillance of rats and gastropods (38) in areas across the continent where the rat lungworm is already established or could potentially become established.
Acknowledgments
We thank all wildlife hospital staff at Consorci per a la Recuperació de la Fauna de les Illes Balears for providing invaluable contributions to establishment of a One Health approach, Sebastià Jaume Ramis for providing help in transport of specimens, and the University of the Balearic Islands for providing support in the establishment of a Biosafety Level 2 laboratory.
This study was partially supported by the Govern de les Illes Balears (grants BIA0920-2 and PDR2020/61).
Biography
Ms. Delgado-Serra is a doctoral student in the Applied Zoology and Animal Conservation Research Group at the University of the Balearic Islands, Palma, Spain. Her primary research interests are the molecular epidemiology of parasites and their vectors in the Mediterranean region.
Footnotes
Suggested citation for this article: Delgado-Serra S, Sola J, Negre N, Paredes-Esquivel C. Angiostrongylus cantonensis nematode invasion pathway, Mallorca, Spain. Emerg Infect Dis. 2022 Jun [_date cited_]. https://doi.org/10.3201/eid2806.212344
1
These authors contributed equally to this article.
References
- 1.McAuliffe L, Fortin Ensign S, Larson D, Bavaro M, Yetto J, Cathey M, et al. Severe CNS angiostrongyliasis in a young marine: a case report and literature review. Lancet Infect Dis. 2019;19:e132–42. 10.1016/S1473-3099(18)30434-1 [DOI] [PubMed] [Google Scholar]
- 2.Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–30. 10.1016/S1473-3099(08)70229-9 [DOI] [PubMed] [Google Scholar]
- 3.Cowie RH. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health. 2013;72(Suppl 2):6–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Sawanyawisuth K, Kitthaweesin K, Limpawattana P, Intapan PM, Tiamkao S, Jitpimolmard S, et al. Intraocular angiostrongyliasis: clinical findings, treatments and outcomes. Trans R Soc Trop Med Hyg. 2007;101:497–501. 10.1016/j.trstmh.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 5.Morgan ER, Modry D, Paredes-Esquivel C, Foronda P, Traversa D. Angiostrongylosis in animals and humans in Europe. Pathogens. 2021;10:1236. 10.3390/pathogens10101236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DY, Stewart TB, Bauer RW, Mitchell M. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol. 2002;88:1024–6. 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 7.McKenzie RA, Green PE, Wood AD. Angiostrongylus cantonesis infection of the brain of a captive Bennett’s wallaby (Macropus rufogriseus). Aust Vet J. 1978;54:86–8. 10.1111/j.1751-0813.1978.tb00354.x [DOI] [PubMed] [Google Scholar]
- 8.Barrett JL, Carlisle MS, Prociv P. Neuro-angiostrongylosis in wild black and grey-headed flying foxes (Pteropus spp). Aust Vet J. 2002;80:554–8. 10.1111/j.1751-0813.2002.tb11039.x [DOI] [PubMed] [Google Scholar]
- 9.Costa LR, McClure JJ, Snider TG III, Stewart TB. Verminous meningoencephalomyelitis by Angiostrongylus (=Parastrongylus) cantonensis in an American miniature horse. Equine Vet Educ. 2000;12:2–6. 10.1111/j.2042-3292.2000.tb01754.x [DOI] [Google Scholar]
- 10.Mason KV, Prescott CW, Kelly WR, Waddell AH. Letter: granulomatous encephalomyelitis of puppies due to Angiostrongylus cantonensis. Aust Vet J. 1976;52:295. 10.1111/j.1751-0813.1976.tb00124.x [DOI] [PubMed] [Google Scholar]
- 11.Monks DJ, Carlisle MS, Carrigan M, Rose K, Spratt D, Gallagher A, et al. Angiostrongylus cantonensis as a cause of cerebrospinal disease in a yellow-tailed black cockatoo (Calyptorhynchus funereus) and two tawny frogmouths (Podargus strigoides). J Avian Med Surg. 2005;19:289–93. 10.1647/2004-024.1 [DOI] [Google Scholar]
- 12.Paredes-Esquivel C, Sola J, Delgado-Serra S, Puig Riera M, Negre N, Miranda MÁ, et al. Angiostrongylus cantonensis in North African hedgehogs as vertebrate hosts, Mallorca, Spain, October 2018. Euro Surveill. 2019;24:1900489. 10.2807/1560-7917.ES.2019.24.33.1900489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HT. A new pulmonary nematode of rats, Pulmonema cantonensis ng, nsp from Canton. Ann Parasitol. 1935;13:312–7. 10.1051/parasite/1935134312 [DOI] [Google Scholar]
- 14.Beaver PC, Rosen L. Memorandum on the first report of Angiostrongylus in man, by Nomura and Lin, 1945. Am J Trop Med Hyg. 1964;13:589–90. 10.4269/ajtmh.1964.13.589 [DOI] [PubMed] [Google Scholar]
- 15.Martín-Carrillo N, Feliu C, Abreu-Acosta N, Izquierdo-Rodriguez E, Dorta-Guerra R, Miquel J, et al. A peculiar distribution of the emerging nematode Angiostrongylus cantonensis in the Canary Islands (Spain): recent introduction or isolation effect? Animals (Basel). 2021;11:1267. 10.3390/ani11051267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowie RH. Angiostrongylus cantonensis: agent of a sometimes fatal globally emerging infectious disease (rat lungworm disease). ACS Chem Neurosci. 2017;8:2102–4. 10.1021/acschemneuro.7b00335 [DOI] [PubMed] [Google Scholar]
- 17.Diao Z, Yin C, Qi H, Wang J. International symposium on Angiostrongylus and angiostrongyliasis, 2010. Emerg Infect Dis. 2011;17:e1. 10.3201/eid1707.102038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foronda P, López-González M, Miquel J, Torres J, Segovia M, Abreu-Acosta N, et al. Finding of Parastrongylus cantonensis (Chen, 1935) in Rattus rattus in Tenerife, Canary Islands (Spain). Acta Trop. 2010;114:123–7. 10.1016/j.actatropica.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Kinsella JM. Angiostrongylus schmidti sp. n. (Nematoda: Metastrongyloidea) from the rice rat, Oryzomys palustris, in Florida, with a key to the species of Angiostrongylus Kamensky, 1905. J Parasitol. 1971;57:494–7. 10.2307/3277901 [DOI] [PubMed] [Google Scholar]
- 20.Monte TC, Simões RO, Oliveira AP, Novaes CF, Thiengo SC, Silva AJ, et al. Phylogenetic relationship of the Brazilian isolates of the rat lungworm Angiostrongylus cantonensis (Nematoda: Metastrongylidae) employing mitochondrial COI gene sequence data. Parasit Vectors. 2012;5:248. 10.1186/1756-3305-5-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Salguero A, Delgado-Serra S, Sola J, Negre N, Miranda MA, Paredes-Esquivel C. Combined morphology and DNA-barcoding to identify Plagiorhynchus cylindraceus cystacanths in Atelerix algirus. Parasitol Res. 2019;118:1473–8. 10.1007/s00436-019-06299-6 [DOI] [PubMed] [Google Scholar]
- 22.Molony SE, Dowding CV, Baker PJ, Cuthill IC, Harris S. The effect of translocation and temporary captivity on wildlife rehabilitation success: an experimental study using European hedgehogs (Erinaceus europaeus). Biol Conserv. 2006;130:530–7. 10.1016/j.biocon.2006.01.015 [DOI] [Google Scholar]
- 23.Halliday JE, Meredith AL, Knobel DL, Shaw DJ, Bronsvoort BM, Cleaveland S. A framework for evaluating animals as sentinels for infectious disease surveillance. J R Soc Interface. 2007;4:973–84. 10.1098/rsif.2007.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spratt M. Neuroangiostrongyliasis disease in wildlife and humans. Microbiol Aust. 2005;26:63. 10.1071/MA05063 [DOI] [Google Scholar]
- 25.Ma G, Dennis M, Rose K, Spratt D, Spielman D. Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet Parasitol. 2013;192:158–65. 10.1016/j.vetpar.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 26.Lunn JA, Lee R, Smaller J, MacKay BM, King T, Hunt GB, et al. Twenty two cases of canine neural angiostrongylosis in eastern Australia (2002-2005) and a review of the literature. Parasit Vectors. 2012;5:70. 10.1186/1756-3305-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason KV. Canine neural angiostrongylosis: the clinical and therapeutic features of 55 natural cases. Aust Vet J. 1987;64:201–3. 10.1111/j.1751-0813.1987.tb15181.x [DOI] [PubMed] [Google Scholar]
- 28.Duffy MS, Miller CL, Kinsella JM, de Lahunta A. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg Infect Dis. 2004;10:2207–10. 10.3201/eid1012.040319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiraoka T, Cuong NC, Hamaguchi S, Kikuchi M, Katoh S, Anh LK, et al. Meningitis patients with Angiostrongylus cantonensis may present without eosinophilia in the cerebrospinal fluid in northern Vietnam. PLoS Negl Trop Dis. 2020;14:e0008937. 10.1371/journal.pntd.0008937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelis S, Spratt DM, Raidal SR. Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland. Aust Vet J. 2011;89:47–50. 10.1111/j.1751-0813.2010.00660.x [DOI] [PubMed] [Google Scholar]
- 31.Martin-Alonso A, Abreu-Yanes E, Feliu C, Mas-Coma S, Bargues MD, Valladares B, et al. Intermediate hosts of Angiostrongylus cantonensis in Tenerife, Spain. PLoS One. 2015;10:e0120686. 10.1371/journal.pone.0120686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Rodríguez S, Puig-Montserrat X. Algerian hedgehog (Atelerix algirus Lereboullet, 1842) habitat selection at the northern limit of its range. Galemys Spanish Journal of Mammalogy. 2014;26:49–56. 10.7325/Galemys.2014.A5 [DOI] [Google Scholar]
- 33.Byers KA, Lee MJ, Patrick DM, Himsworth CG. Rats about town: a systematic review of rat movement in urban ecosystems. Front Ecol Evol. 2019;7:13. 10.3389/fevo.2019.00013 [DOI] [Google Scholar]
- 34.Segade P, García-Estévez J, Arias C, Iglesias R. Parasitic infections in mixed system-based heliciculture farms: dynamics and key epidemiological factors. Parasitology. 2013;140:482–97. 10.1017/S0031182012001795 [DOI] [PubMed] [Google Scholar]
- 35.Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, Eberhard ML, et al. Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis. 2002;8:324–6. 10.3201/eid0803.010316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al Hammoud R, Nayes SL, Murphy JR, Heresi GP, Butler IJ, Pérez N. Angiostrongylus cantonensis meningitis and myelitis, Texas, USA. Emerg Infect Dis. 2017;23:1037–8. 10.3201/eid2306.161683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flerlage T, Qvarnstrom Y, Noh J, Devincenzo JP, Madni A, Bagga B, et al. Angiostrongylus cantonensis eosinophilic meningitis in an infant, Tennessee, USA. Emerg Infect Dis. 2017;23:1756–8. 10.3201/eid2310.170978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stockdale Walden HD, Slapcinsky JD, Roff S, Mendieta Calle J, Diaz Goodwin Z, Stern J, et al. Geographic distribution of Angiostrongylus cantonensis in wild rats (Rattus rattus) and terrestrial snails in Florida, USA. PLoS One. 2017;12:e0177910. 10.1371/journal.pone.0177910 [DOI] [PMC free article] [PubMed] [Google Scholar]