Subunit II of Bacillus subtilis Cytochrome c Oxidase Is a Lipoprotein (original) (raw)
Abstract
The sequence of the N-terminal end of the deduced ctaC gene product of Bacillus species has the features of a bacterial lipoprotein. CtaC is the subunit II of cytochrome _caa_3, which is a cytochrome c oxidase. Using Bacillus subtilis mutants blocked in lipoprotein synthesis, we show that CtaC is a lipoprotein and that synthesis of the membrane-bound protein and covalent binding of heme to the cytochrome c domain is not dependent on processing at the N-terminal part of the protein. Mutants blocked in prolipoprotein diacylglyceryl transferase (Lgt) or signal peptidase type II (Lsp) are, however, deficient in cytochrome _caa_3 enzyme activity. Removal of the signal peptide from the CtaC polypeptide, but not lipid modification, is seemingly required for formation of functional enzyme.
The gram-positive bacterium Bacillus subtilis contains at least three different respiratory oxidases in the cytoplasmic membrane: two quinol oxidases, cytochrome _aa_3 and cytochrome bd, and one cytochrome c oxidase, cytochrome _caa_3 (24). Studies of mutants have shown that, under aerobic conditions, cytochrome _aa_3 is the major oxidase, and a deficiency in this enzyme significantly affects cell growth (16). In contrast, lack of cytochrome _caa_3 has little effect on growth of B. subtilis (21). Cytochrome _caa_3 oxidase activity is required for oxidation of N,N,N′,N′-tetramethyl-_p_-phenylenediamine (TMPD), a property which can be used to identify mutants defective in assembly or function of the oxidase (21).
In bacteria, lipoproteins are membrane bound. The lipid is covalently attached to a cysteine residue at the N-terminal end. The synthesis of lipoproteins occurs in two steps (for a review, see reference 4). First, a diacylglyceryl moiety is attached to the sulfur of the cysteine residue of the prolipoprotein (the prolipoprotein contains a signal sequence at its N-terminal end). This step is catalyzed by prolipoprotein diacylglyceryl transferase, encoded by the lgt gene. In the second step, signal peptidase type II, which is specific for lipoprotein maturation and encoded by the lsp gene, removes the signal sequence by cleaving the peptide bond on the N-terminal side of the diacylglyceride-cysteine residue. Generally the N terminus is finally acylated by the action of the apolipoprotein _N_-acyltransferase, encoded by the lnt gene. The B. subtilis genome contains genes for many (>100) different putative lipoproteins and one lgt and one lsp gene, but an lnt gene has not been found. Only a few lipoproteins have so far been experimentally identified in B. subtilis, e.g., PrsA, OpuAC, and KapB (3, 8, 9).
During work with B. subtilis Lgt- and Lsp-deficient mutants, we observed that they are deficient in oxidizing TMPD, suggesting an effect of the mutations on cytochrome _caa_3. The subunit polypeptides of cytochrome _caa_3 are encoded by the ctaCDEF genes (17). Subunit I (CtaD) harbors the two heme A groups. Subunit II (CtaC) contains the dicopper center, CuA, and has a cytochrome c domain at the C-terminal end. The CuA and cytochrome c domains are exposed at the outer side of the cytoplasmic membrane. Subunit II is anchored in the membrane by two transmembrane α-helical segments constituted by the N-terminal half of the polypeptide, as inferred from its close similarity to Paracoccus denitrificans cytochrome _aa_3, whose three-dimensional structure has been determined (7). Thus, both the N- and C-terminal ends of the mature protein are located on the outer side of the cytoplasmic membrane. The deduced B. subtilis CtaC (pro)-polypeptide consists of 356 amino acid residues, and the first half contains three segments with hydrophobic residues. The most N-terminal segment is absent in the assembled oxidase of Bacillus sp. strain PS3 (6), and it has the features of a signal sequence typical of lipoproteins (a lipo box: -_Leu_-Ala/Ser-Gly/Ala-_Cys_-), with a cysteine residue at the predicted cleavage site (position 21) (Fig. 1). The possibility that CtaC of Bacillus species is a lipoprotein was previously noted by Quirk et al. (13). In this report, we show that subunit II of B. subtilis cytochrome _caa_3 is a lipoprotein and demonstrate that covalent binding of heme to the CtaC polypeptide and assembly of cytochrome _caa_3 proceed in mutants blocked in lipoprotein synthesis.
FIG. 1.
Deduced N-terminal sequence of subunit II polypeptides of three oxidases. CtaC, QoxA, and CyoA are subunits of cytochrome _caa_3, cytochrome _aa_3, and cytochrome _bo_3, respectively, of the specified bacteria. The signal peptidase type II signal cleavage site, indicated with an arrow, is supported by experimental data obtained for Bacillus sp. strain PS3 (6), Bacillus subtilis W23 (10), and E. coli (11).
In vivo labeling of Lgt- and Lsp-deficient mutants with radioactive palmitic acid.
We have made use of three mutations that affect lipoprotein synthesis: an lgt insertion where the downstream genes (yvoD, yvoE, and yvoF) are transcribed from the P_spac_ promoter to avoid polar effects from the insertion (strains with this mutation were grown in the presence of 1 mM isopropyl-β-d-thiogalactopyranoside [IPTG] except in the experiment represented by Table 2), an lsp deletion, and an lsp insertion where the intact lsp gene is transcribed from the P_spac_ promoter (i.e., transcription of lsp is inducible by IPTG) (12). The constructions of the Lgt and Lsp knockout mutants (Table 1) are described elsewhere (15, 19a).
TABLE 2.
TMPD oxidation activity of colonies on plates
| Strain | Relevant genotype | Growth mediuma | |||
|---|---|---|---|---|---|
| TBAB plates | NSMP plates | ||||
| +IPTG | −IPTG | +IPTG | −IPTG | ||
| 168A | Wild type | ++++ | ++++ | ++++ | ++++ |
| LUH15 | ctaCD | − | − | − | − |
| LUH104 | lgt | − | − | ++ | + |
| LUH102 | lsp | − | − | − | − |
| LUH103 | lsp under IPTG-inducible promoter | +++ | − | +++ | − |
TABLE 1.
B. subtilis strains used in this work
| Strain | Genotype | Origin or referencea |
|---|---|---|
| 168A | trpC2 | 25 |
| 8G5MI_lsp_ | _trp tyr met his ade ura nic rib lsp_ΩpMUTIN2(ery) | 19a |
| 8G5MΔ_lsp_ | trp tyr met his ade ura nic rib Δlsp::pMUTIN2(ery) | 19a |
| JO1 | trpC2 ade met ΔctaCD::ble | 21 |
| L16220 | trpC2 phe-1 lgt_ΩpCR_lauC (ery) | 15 |
| LUH14 | trpC2 ΔqoxABCD::kan | Δqox→168A |
| LUH15 | trpC2 ΔctaCD::ble | JO1→168A |
| LUH17 | trpC2 ΔctaCD::ble ΔqoxABCD::kan | Δqox→LUH15 |
| LUH102 | trpC2 Δlsp::pMUTIN2(ery) | 8G5MΔ_lsp_→168A |
| LUH103 | _trpC2 lsp_ΩpMUTIN2(ery) | 8G5MI_lsp_→168A |
| LUH104 | trpC2 lgt_Ω pCR_lauC(ery) | L16220→168A |
| LUH108 | trpC2 ΔqoxABCD::kan Δlsp:: pMUTIN2(ery) | 8G5MΔ_lsp_→LUH14 |
| LUH109 | trpC2 ΔqoxABCD::_kan lsp_Ω pMUTIN2(ery) | 8G5MI_lsp_→LUH14 |
| LUH110 | trpC2 ΔqoxABCD::kan lgt_Ω pCR_lauC(ery) | L16220→LUH14 |
| Δqox | trpC2 ΔqoxABCD::kan | 23 |
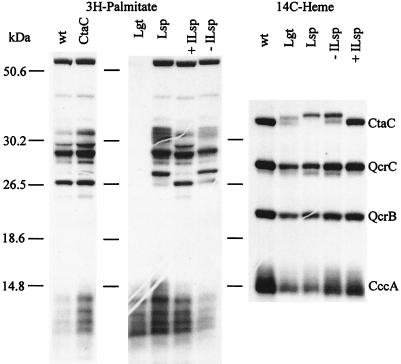
To radiolabel lipoproteins, strains with the respective mutations (LUH104, LUH102, and LUH103) and the parental strain (168A) (Table 1) were grown in nutrient sporulation medium with phosphate (NSMP) (5) supplemented with 80 nM [9,10(n)-3H]palmitic acid (51 Ci/mmol) (Amersham). In contrast to Escherichia coli, B. subtilis can grow in the absence of functional Lgt or Lsp. Membranes were isolated as previously described (19), lipids were extracted (4), and lipoproteins were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography (Fig. 2). Membranes of the wild type showed four major and many minor radioactive polypeptide bands. As expected, the Lgt knockout mutant LUH104, blocked in the first step of the lipoprotein biosynthetic pathway, lacked these bands; the radiolabel observed as a ladder at the front of the gel corresponds to free lipid- and low-molecular-weight lipid-containing compounds such as glycolipids. The band patterns of the Lsp deletion mutant LUH102 and mutant LUH103 with the inducible lsp gene grown in the absence of IPTG differed from those of 168A and LUH103 grown in the presence of IPTG. For example, a major radioactive polypeptide of about 27 kDa migrated more slowly in the Lsp-deficient mutants, suggesting that the signal-peptide has not been removed. A distinct radiolabeled polypeptide in the 38-kDa region possibly corresponding to CtaC could not be detected; i.e., all bands observed in the wild type were also found in strain LUH15 in which the ctaCD genes are absent (Fig. 2). However, it should be noted that the CtaC polypeptide constitutes less than 0.1% of the total membrane protein content of wild-type cells grown in NSMP (21), a quantity that might be below the detection level in this type of experiment.
FIG. 2.
Analysis of B. subtilis strains for membrane-bound lipoproteins and covalently bound heme by [3H]palmitate and [14C]ALA (heme) labeling, respectively. Fluorographs (2) of SDS gels (16% [wt/vol] acrylamide) (18) are shown. Growth conditions and preparation of extracts were as described in the text. Forty micrograms of membrane protein was loaded in each lane. The samples were as follows: wt, strain 168A; CtaC, LUH15; Lgt, LUH104; Lsp, LUH102; ILsp, LUH103. + and − indicate that the extract was prepared from cells grown in the presence or absence, respectively, of 1 mM IPTG. The numbers on the left indicate the positions of size markers. The specific 14C label in heme in the Lgt- and Lsp-deficient mutants was much lower than in the wild type for reasons that are discussed in the text. The fluorographs shown are overexposed to demonstrate that lipoproteins are absent from strain LUH104 and to more clearly show the CtaC polypeptide(s) in mutants defective in lipoprotein modification. The amount of CtaC polypeptide in LUH102 and LUH104 was about 60% of that in the wild type, as calculated from the [14C]heme in CtaC relative to that in QcrC; i.e., heme in QcrC was used as an internal standard with the assumption that the amount of QcrC polypeptide was the same in membranes from all strains.
Cytochromes c in mutants blocked in lipoprotein modification.
Cytochromes of the c type can be analyzed by growing cells in the presence of radioactive 5-aminolevulinic acid (ALA)—a heme biosynthetic precursor—and by SDS-PAGE of extracts followed by autoradiography (19). In contrast to other heme proteins, heme in cytochrome c is covalently bound to the polypeptide and therefore remains attached to polypeptide after electrophoresis in the presence of SDS. Membranes of B. subtilis wild-type strains contain four main proteins with covalently bound heme: CtaC, QcrC, QcrB, and CccA. The QcrC and QcrB polypeptides are subunits of the cytochrome bc complex, and CccA is cytochrome _c_550 (21, 25).
All four cytochromes were found in membranes of Lgt- and Lsp-deficient mutants, as determined by labeling with [14C]ALA (Fig. 2). However, the Lgt and Lsp knockout mutants as well as the mutant with the inducible lsp gene grown without IPTG exhibited two differences compared to the wild type and the latter mutant grown in the presence of IPTG. (i) The incorporation of radioactivity into different cytochrome polypeptides was lower (e.g., compare QcrB band intensities in samples wt and Lgt), a phenomenon probably due to a deficiency in uptake of [14C]ALA. ALA is taken up by dipeptide transport systems (22). Polypeptide components of these transporters in B. subtilis are predicted lipoproteins (AppA, DciAE, and OppA) and might be defective in Lgt- and Lsp-deficient mutants. Furthermore, cytochrome _c_550 is sensitive to proteolysis, and the weak CccA band of the mutants may be a result of increased proteolytic activity, as previously observed with certain B. subtilis strains (26). (ii) The migration of the CtaC polypeptide in the gel varied, indicating size differences. CtaC in the Lgt knockout mutant appeared as a 39- and a 38-kDa polypeptide, the latter being the same size as the mature wild-type protein. The observation of the larger polypeptide is consistent with the presence of the 20-residue signal peptide expected in the Lgt-deficient mutant if CtaC is a lipoprotein. The form of the protein that migrated at the position of wild-type CtaC is most likely due to removal of the signal peptide from a fraction of the population of propolypeptide, possibly catalyzed by one or more of the five type I signal peptidases present in B. subtilis (20). CtaC from the Lsp knockout mutant appeared as a single 40-kDa band, i.e., migrating more slowly than both CtaC polypeptides present in the Lgt-deficient mutant. This is in agreement with a lack of signal peptidase type II; i.e., the polypeptide is lipid modified but the signal peptide is not removed. Some CtaC migrating as mature, wild-type CtaC was observed in the mutant with the inducible lsp gene grown in the absence of added IPTG. This can be explained by the low level of transcription of the lsp gene in the absence of added inducer. From the electrophoretic mobility of the CtaC polypeptide synthesized in Lgt- and Lsp-deficient mutants, we conclude that subunit II of cytochrome _caa_3 is a lipoprotein.
Role of lipid modification of subunit II.
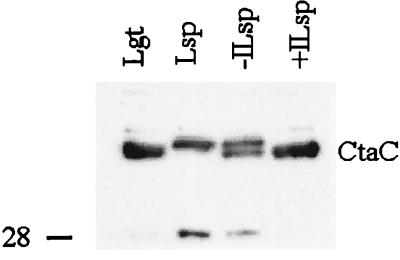
The analysis of cytochromes c by radioactive labeling demonstrated that the CtaC polypeptide in Lgt- and Lsp-deficient mutants contains covalently bound heme, i.e., that the cytochrome c domain is assembled and located on the outer side of the membrane. However, the relative amount of CtaC compared to QcrC and QcrB, as estimated from the heme content (radioactivity), was significantly lower in the mutants. Immunoblot analysis with antibodies raised against the C-terminal peptide of CtaC (Fig. 3) showed that this difference was due to decreased amounts of the CtaC polypeptide in membranes of the mutants rather than to a deficiency in the synthesis of cytochrome c. An autoradiogram to detect 14C on the blotted membrane (not shown) showed a pattern with relative intensities of bands identical to that of the immunoblot. The 40-kDa form of Cta in the Lsp-deficient mutant membranes was not transferred from the acrylamide gel to the immunoblot membrane as efficiently as the 38-kDa form, accounting for the difference in relative band intensities in Fig. 2 and 3.
FIG. 3.
Immunoblot analysis for CtaC polypeptide in membranes. The samples are the same as those analyzed for covalently bound heme in Fig. 2. SDS-PAGE was carried out as described for Fig. 2 except that about 35 μg of protein was loaded in each lane. This gel did not resolve the two forms of CtaC in the Lgt-deficient mutant. Proteins were electroblotted onto a polyvinylidene difluoride membrane under semidry conditions with Tris-glycine buffer with 20% methanol. The anti-CtaC serum, used at a 500-fold dilution, had been obtained by immunizing a rabbit with the peptide MLNALTEKRTRGC, corresponding to the C-terminal end of B. subtilis CtaC, conjugated to bovine serum albumin. The ECL Western blot system (Amersham) was used to detect antigens. The position of a 28-kDa size marker is indicated on the left.
The immunoblot analysis also showed that a heme-containing polypeptide of about 28 kDa (migrating just in front of QcrC) present in the Lgt- and Lsp-deficient mutants is a fragment of CtaC. The apparent size of this fragment, its localization in the membrane, and its reactivity with the peptide antiserum suggest that it corresponds to CtaC polypeptide truncated at the N-terminal end but containing at least one transmembrane segment.
As mentioned, colonies of Lgt- and Lsp-deficient mutants have reduced TMPD oxidation activity (Table 2). This phenotype was more pronounced in colonies grown on Tryptose blood agar base (TBAB) (Difco) plates than in those grown on NSMP plates. Probably, the steady-state amount of cytochrome _caa_3 is higher in cells grown on NSMP than on TBAB. To determine the activity of cytochrome _caa_3 in Lgt- and Lsp-deficient mutants, cytochrome c oxidation activity of isolated membranes (from cells grown in NSMP in the presence of IPTG) was measured spectrophotometrically as described before (21) except that we used 20 μM reduced Saccharomyces cerevisiae cytochrome c as a substrate. Membranes of the Lgt (LUH104)- and Lsp (LUH102)-deficient mutants contained 26% ± 8% and 5% ± 2% of the cytochrome c oxidase activity, respectively, of the parental strain 168A (0.20 μmol of cytochrome c oxidized per mg of membrane protein per min). Membranes of strain LUH15, devoid of cytochrome _caa_3, showed no cytochrome c oxidase activity. The results demonstrate that a deficiency in Lsp has a much more drastic effect on cytochrome _caa_3 activity than a deficiency in Lgt.
To analyze the amount of assembled cytochrome _caa_3 in mutants defective in lipoprotein synthesis, we constructed Lgt- and Lsp-deficient strains also lacking the major cytochrome a, i.e., cytochrome _aa_3, encoded by the qoxABCD operon. The different strains (LUH108, LUH109, and LUH110) as well as the control, strain LUH17 lacking both cytochrome _aa_3 and cytochrome _caa_3, were grown in NSMP medium to the end of the exponential growth phase. In these strains, cytochrome bd functions as a terminal oxidase to support growth. Isolated membranes (5) were analyzed for cytochrome a by light absorption spectroscopy (19). Only an assembled cytochrome _caa_3 containing both subunits I and II shows absorption at 605 nm in ascorbate- or dithionite-reduced minus ferricyanide-oxidized difference spectra. The mutants lacking Lgt or Lsp contained approximately 50% of the wild-type level of cytochrome a. This amount correlated with the relative amounts of CtaC polypeptide, determined by immunoblot analysis of membrane preparations (spectra and blot not shown) and calculated from [14C]heme (Fig. 2), indicating that most of the CtaC polypeptides in the membrane were present in assembled enzyme.
Conclusion.
Cytochrome _caa_3 belongs to a family of heme-copper containing oxidases that includes cytochrome c oxidases and quinol oxidases (1). Based on the content of metal centers and the sequence in the C-terminal half of the polypeptide, subunit II proteins fall into three classes: those that contain the CuA center (e.g., cytochrome _aa_3 of mammalian mitochondria and P. denitrificans); those that contain both CuA and a cytochrome c domain (e.g., cytochrome _caa_3 of Bacillus species); and those that lack metal centers (e.g. E. coli cytochrome _bo_3). Subunit II of various oxidases in the family can now be further classified according to the chemical composition of its N-terminal end. It was recently demonstrated that subunit II (CyoA) of E. coli cytochrome _bo_3 is a lipoprotein (11), and in this work we show that subunit II (CtaC) of B. subtilis cytochrome _caa_3 is a lipoprotein.
The N-terminal sequence of CyoA, like those of CtaC from several Bacillus species, has a motif characteristic of lipoproteins. This motif is also present in the N-terminal sequence of B. subtilis QoxA, subunit II of cytochrome _aa_3 (Fig. 1). Functional cytochrome _aa_3 is assembled in B. subtilis Lgt- and Lsp-deficient mutants, as judged from growth properties and cytochrome absorption spectra (data not shown). The presequence of subunit II of P. denitrificans cytochrome c oxidase (cytochrome _aa_3), which clearly is not a lipoprotein, does not contain a lipobox. Intriguingly, the N-terminal sequence of subunit II of P. denitrificans quinol oxidase (cytochrome _ba_3) is processed and the mature protein contains a cysteine residue at its N-terminus, but it is not modified by lipid (14, 27), showing that lipoproteins cannot be predicted with certainty from sequence data.
Lipid modification of subunit II of E. coli cytochrome _bo_3 is not required for assembly or function of the oxidase, since replacement of the relevant cysteine residue by serine has no drastic effect (11). In the case of B. subtilis CtaC, posttranslational modification at the N-terminal end does not seem to be required for assembly but may be important for oxidase activity. The Lgt and Lsp knockout mutants both contained about 50% cytochrome _caa_3 chromophore in membranes compared to the wild type but showed ca. 26% and only ca. 5% cytochrome c oxidase activity, respectively. The activity in the Lgt mutant may be explained by the removal of the signal peptide from a fraction (about 50%) of the CtaC polypeptides. Thus, it seems as though removal of the signal peptide from, but not the lipid modification of, subunit II is required for the formation of active cytochrome _caa_3 in B. subtilis. Membranes of strain LUH103 with the inducible lsp gene grown in liquid medium without IPTG contained some 38-kDa CtaC (without signal peptide) (Fig. 2) but were TMPD oxidation negative on plates. This difference can be explained by the different growth conditions, i.e., that little of the 38-kDa form is formed in colonies.
Acknowledgments
We are grateful to Matti Saraste for providing antiserum against CtaC.
This work was supported by grants from the Swedish Natural Science Research Council to L.H.; by grants from Genencor International (Rijswijk, The Netherlands) and Gist-brocades BV (Delft, The Netherlands) to H.T., Sierd Bron, and Jan Maarten van Dijl; and by grant 96.0245 from the Office Fédéral de l’Education et de la Science (Switzerland) to Dimitri Karamata.
REFERENCES
- 1.Calhoun M W, Thomas J W, Gennis R B. The cytochrome oxidase superfamily of redox-driven proton pumps. Trends Biochem Sci. 1994;19:325–330. doi: 10.1016/0968-0004(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain J P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979;98:132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- 3.Dartois V, Djavakhishvili T, Hoch J A. KapB is a lipoprotein required for KinB signal transduction and activation of the phosphorelay to sporulation in Bacillus subtilis. Mol Microbiol. 1997;26:1097–1108. doi: 10.1046/j.1365-2958.1997.6542024.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi S, Wu H C. Identification and characterization of lipid-modified proteins in bacteria. In: Hooper N M, Turner A J, editors. Lipid modification of proteins. Oxford, England: IRL Press; 1992. pp. 261–285. [Google Scholar]
- 5.Hederstedt L. Molecular properties, genetics, and biosynthesis of Bacillus subtilis succinate dehydrogenase complex. Methods Enzymol. 1986;126:399–414. doi: 10.1016/s0076-6879(86)26040-1. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka M, Machida K, Shimada S, Mogi A, Tsuchiya T, Ohmori T, Souma Y, Gonda M, Sone N. Nucleotide sequence of the gene coding for four subunits of cytochrome c oxidase from the thermophilic bacterium PS3. J Biochem. 1990;108:866–873. doi: 10.1093/oxfordjournals.jbchem.a123294. [DOI] [PubMed] [Google Scholar]
- 7.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 8.Kempf B, Gade J, Bremer E. Lipoprotein from the osmoregulated ABC transport system OpuA of Bacillus subtilis: purification of the glycine betaine binding protein and characterization of a functional lipidless mutant. J Bacteriol. 1997;179:6213–6220. doi: 10.1128/jb.179.20.6213-6220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontinen V P, Sarvas M. The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol Microbiol. 1993;8:727–737. doi: 10.1111/j.1365-2958.1993.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 10.Lemma E, Schägger H, Kröger A. The menaquinol oxidase of Bacillus subtilis W23. Arch Microbiol. 1993;159:574–578. doi: 10.1007/BF00249037. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Katsonouri A, Gennis R B. Subunit II of the cytochrome bo3 ubiquinol oxidase from Escherichia coli is a lipoprotein. Biochemistry. 1997;36:11298–11303. doi: 10.1021/bi9709710. [DOI] [PubMed] [Google Scholar]
- 12.Prági Z, Tjalsma H, Bolhuis A, van Dijl J M, Venema G, Bron S. The signal peptidase II (lsp) gene of Bacillus subtilis. Microbiology. 1997;143:1327–1333. doi: 10.1099/00221287-143-4-1327. [DOI] [PubMed] [Google Scholar]
- 13.Quirk P G, Hicks D B, Krulwich T A. Cloning of the cta operon from the alkaliphilic Bacillus firmus OF4 and characterization of the pH-regulated cytochrome caa3 oxidase it encodes. J Biol Chem. 1993;268:678–685. [PubMed] [Google Scholar]
- 14.Richter O-M H, Tao J, Turba A, Ludwig B. A cytochrome ba3 functions as a quinol oxidase in Paracoccus denitrificans. J Biol Chem. 1994;269:23079–23086. [PubMed] [Google Scholar]
- 15.Robinson C, Rivolta C, Karamata D, Moir A. The product of the yvoC (gerF) gene of Bacillus subtilis is required for spore germination. Microbiology. 1998;144:3105–3109. doi: 10.1099/00221287-144-11-3105. [DOI] [PubMed] [Google Scholar]
- 16.Santana M, Kunst F, Hullo M F, Rapoport G, Danchin A, Glaser P. Molecular cloning, sequencing and physiological characterisation of the qox operon from Bacillus subtilis encoding the aa3-600 quinol oxidase. J Biol Chem. 1992;267:10225–10231. [PubMed] [Google Scholar]
- 17.Saraste M, Metso T, Nakari T, Jalli T, Lauraeus M, van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved theme. Eur J Biochem. 1991;195:517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- 18.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 19.Schiött T, von Wachenfeldt C, Hederstedt L. Identification and characterization of the ccdA gene required for cytochrome c synthesis in Bacillus subtilis. J Bacteriol. 1997;179:1962–1973. doi: 10.1128/jb.179.6.1962-1973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Tjalsma, H., and J. M. van Dijl. Unpublished data.
- 20.Tjalsma H, Noback M A, Bron S, Venema G, Yamane K, van Dijl J M. Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities: constitutive and temporally controlled expression of different sip genes. J Biol Chem. 1997;272:25983–25992. doi: 10.1074/jbc.272.41.25983. [DOI] [PubMed] [Google Scholar]
- 21.van der Oost J C, von Wachenfeldt C, Hederstedt L, Saraste M. Bacillus subtilis cytochrome oxidase mutants: biochemical analysis and genetic evidence for two aa3-type oxidases. Mol Microbiol. 1991;5:2063–2072. doi: 10.1111/j.1365-2958.1991.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 22.Verkamp E, Backman V M, Björnsson J M, Söll D, Eggertsson G. The periplasmic dipeptide permease system transports 5-aminolevulinic acid in Escherichia coli. J Bacteriol. 1993;175:1452–1456. doi: 10.1128/jb.175.5.1452-1456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villani G, Tattoli M, Capitanio N, Glaser P, Papa S, Danchin A. Functional analysis of subunits III and IV of Bacillus subtilis aa3-600 quinol oxidase by in vitro mutagenesis and gene replacement. Biochim Biophys Acta. 1995;1232:67–74. doi: 10.1016/0005-2728(95)00112-5. [DOI] [PubMed] [Google Scholar]
- 24.von Wachenfeldt C, Hederstedt L. Molecular biology of Bacillus subtilis cytochromes. FEMS Microbiol Lett. 1992;100:91–100. doi: 10.1111/j.1574-6968.1992.tb14025.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Le Brun N E. Studies of the cytochrome subunits of menaquinone:cytochrome c reductase (bc complex) of Bacillus subtilis. J Biol Chem. 1998;273:8860–8866. doi: 10.1074/jbc.273.15.8860. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Hederstedt L, Piggot P J. The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J Bacteriol. 1995;177:6751–6760. doi: 10.1128/jb.177.23.6751-6760.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zickermann I, Anemüller S, Richter O-M H, Tautu O S, Link T A, Ludwig B. Biochemical and spectroscopic properties of the four-subunit quinol oxidase (cytochrome ba3) from Paracoccus denitrificans. Biochim Biophys Acta. 1996;1277:93–102. doi: 10.1016/s0005-2728(96)00086-2. [DOI] [PubMed] [Google Scholar]