Neuroangiostrongyliasis: Global Spread of an Emerging Tropical Disease (original) (raw)
ABSTRACT.
Neuroangiostrongyliasis (NAS) is an emerging parasitic disease caused by the neurotropic nematode Angiostrongylus cantonensis. Since it was first discovered, in rats in southern China in the 1930s, this tropical to subtropical parasite has spread to much of Southeast Asia, the Pacific Islands (including Hawaii), Australia, Japan, South America, the southeastern United States, the Caribbean, Africa, the Canary Islands, and the Balearic Islands. The parasite completes its natural life cycle in snails and slugs (intermediate hosts), and rats (definitive hosts). Humans become accidental hosts after ingesting infective third-stage larvae contained within uncooked or undercooked intermediate or paratenic hosts, an event that sometimes results in NAS, also known as rat lungworm disease. Although A. cantonensis larvae cannot complete their life cycle in humans, their migration into the brain and spinal cord combined with a powerful inflammatory reaction often leads to eosinophilic meningitis and can, in rare instances, lead to coma, paralysis, and death or, in other cases, chronic, disabling neurologic sequelae. Symptoms of NAS are diverse, which often makes it difficult to diagnose. Treatment may include administration of analgesics, corticosteroids, anthelminthics, and repeat lumbar punctures to reduce intracranial pressure. Unfortunately, few medical providers, even in endemic areas, are familiar with A. cantonensis or its epidemiology, diagnosis, and treatment. As the parasite continues to spread and NAS affects more people, medical practitioners, as well as the general public, must become more aware of this emerging zoonosis and the potentially devastating harm it can cause.
INTRODUCTION
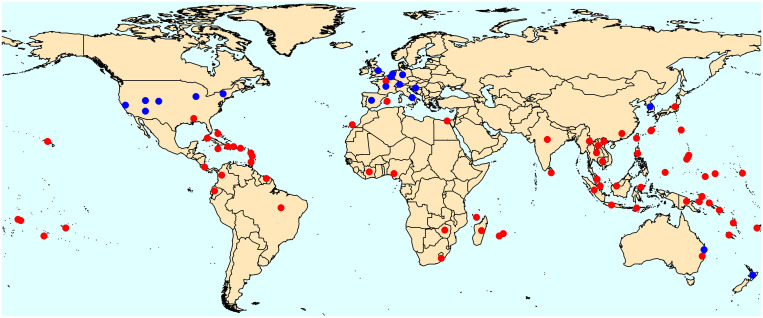
Angiostrongylus cantonensis was first discovered in rats in southern China in 1933; this area is generally accepted as the parasite’s region of origin. The first known human case of neuroangiostrongyliasis (NAS) was identified in nearby Taiwan in 1944, although appreciated only after the parasite and the disease were clearly linked almost two decades later.1 From this region, the parasite spread westward through Southeast Asia, eastward to islands of the Pacific, north to Japan, and south to Australia, no doubt associated with military movements during and immediately after World War II, and increasing travel and trade during the latter half of the 20th century.2 Angiostrongylus cantonensis was then found in a number of Caribbean islands and in the southeastern United States, where it was first reported in 1988,3 and more recently in South America4,5 (Figure 1). Reports of NAS often precede detection of A. cantonensis in regional faunas, as likely animal hosts are rarely screened until a human case arises. Nonetheless, A. cantonensis has most recently been found in rodents in the Canary Islands19 and hedgehogs in the Balearic Islands of the Mediterranean,14 where NAS has not yet been reported. Human cases are also rising in travelers returning from endemic regions,12,16,17 and there has even been an enigmatic case in northern France that did not involve travel or known ingestion of imported food.16 This review takes a global perspective, while focusing in somewhat greater detail on the United States, including Hawaii.
Figure 1.
The global distribution, by country, of combined reported presence of Angiostrongylus cantonensis and cases of human or animal neuroangiostrongyliasis (red circles), with cases in returning travelers to nonendemic areas distinguished (blue circles).2,4–19 If part of a country is distant from the main part of the country—Guam, Saipan, Hawaii, American Samoa, and Puerto Rico (United States); Ryukyu and Ogasawara Islands (Japan); New Caledonia, Tahiti, Mayotte, Réunion, Guadeloupe, and Martinique (France); and Canary Islands and Mallorca (Spain)—dots are placed on those locations in addition to the main part of country (if also present there). For most countries/territories, a single dot has been placed roughly where the greatest concentration of records occurs. For countries with records on multiple widespread islands, dots are placed on those areas with records—Malaysia (Peninsula Malaysia, Sarawak), Indonesia (Java, Sumatra, Flores, Sulawesi), and the Federated States of Micronesia (Chuuk, Pohnpei). For the continental United States, several dots for returning travelers are placed roughly where they were diagnosed. Note that the cases in the Bahamas may have originated in Louisiana, and the presence of A. cantonensis in Zimbabwe is unconfirmed.
INFECTION PATHWAYS
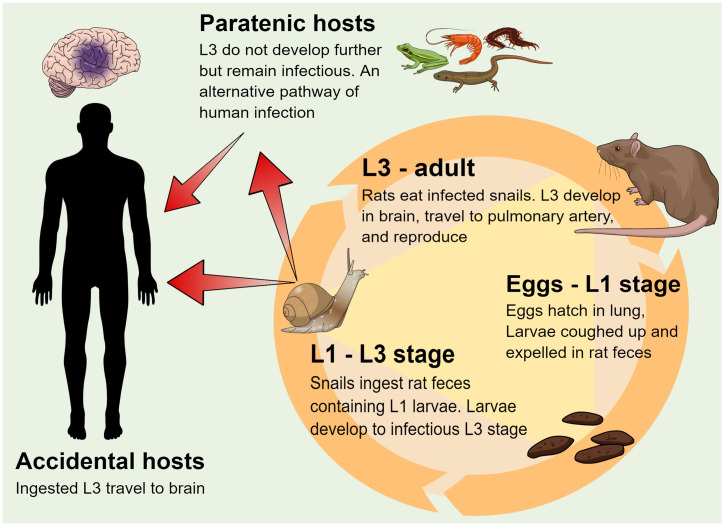
To understand the parasite’s transmission to humans, one must understand its life cycle (Figure 2). Rats (definitive hosts) and snails and slugs (intermediate hosts; hereafter “snails”) are both required to complete the natural life cycle.9 Paratenic hosts (e.g., freshwater prawns, frogs and toads, and land crabs, with centipedes most recently reported),22 in which the infectious stage 3 larvae (L3) are acquired by ingestion of intermediate hosts or other paratenic hosts carrying L3, do not support development of the L3. However, paratenic hosts, in which the L3 remain dormant, can still infect accidental hosts (Figure 2). Humans, and various other animals, are accidental hosts in which the infectious L3 can neither develop beyond the subadult stage (L5) nor reproduce.9 Instead, after accidental hosts acquire L3 by ingesting infected intermediate or paratenic hosts, their L3 quickly reach the central nervous system, primarily the brain, where they feed, grow, molt, and eventually die, as opposed to returning to the pulmonary artery to reproduce, as would normally occur in a rat (Figure 2).
Figure 2.
Angiostrongylus cantonensis completes its life cycle in various species of rats (definitive hosts) and snails (intermediate hosts).9,20,21 Snails become infected by ingesting rat feces containing freshly hatched larvae (L1). The larvae develop into infectious third-stage larvae (L3) in the snail and remain as such for the life span of the snail or until the snail is eaten by a definitive, paratenic, or accidental host.
Raw or poorly cooked intermediate or paratenic hosts carrying L3 are the classic vehicles of human infection. Raw snails are sometimes eaten on a dare or for a bet, as reported in Louisiana,23 Hawaii,24,25 Okinawa,26 New Caledonia,27 Australia,28,29 and Brazil (S. Thiengo, personal communication). Raw or undercooked snails are also eaten as delicacies, especially in parts of China and Thailand.30,31 Among paratenic hosts,22 freshwater prawns are widely eaten raw, especially in French Polynesia, where they have been heavily implicated in epidemics of NAS since the 1950s.32 The liver, meat, tongue, and testes of monitor lizards are eaten in India, Sri Lanka, Thailand, and Laos, primarily by men who believe these organs will improve their strength and virility.33–35 Other paratenic hosts eaten raw include land crabs and centipedes,32,36 as well as frogs and toads sometimes consumed for health purposes,37 and in one case in Louisiana on a dare.38 Obtaining a history of eating one of these intermediate or paratenic hosts raw can facilitate an early diagnosis of NAS.
However, in the absence of other clues, inadvertent ingestion of intermediate hosts (snails)—in particular, a whole or partial snail hidden in produce, especially leafy greens—is often the likeliest pathway of infection. In recent years, raw, blended vegetable juice (including so-called “green smoothies”) contaminated with infective larvae from snails accidentally blended along with the vegetables has been identified as another important source of infection.39 Only in rare cases, however, is there good circumstantial evidence for this; for example, among a group of students returning to the United States from a vacation in Jamaica, those who had eaten a Caesar salad on their last evening became ill with NAS, whereas those who had not done so remained uninfected and healthy.40 Even more rarely is there definitive proof. Nonetheless, inadvertent ingestion of intermediate hosts is probably the most important means of infection in many regions, including Hawaii. Paratenic hosts—namely, flatworms41,42—may also contaminate fresh leafy greens and other produce.
Additional infection pathways have been suggested.32 For example, can one become infected with A. cantonensis L3 by ingesting the slime trail laid down by an infected snail on a lettuce leaf? Several studies addressing this question have in fact demonstrated little or no release of L3 in slime.43 In Hawaii, some researchers have speculated that snails that drown in rainfall catchment tanks may release sufficient numbers of L3 to cause NAS in individuals who drink the contaminated water.44 Although large numbers of L3 have been observed leaving dead and dying snails (R. L. Rollins and R. H. Cowie, unpublished data), there are no data on the prevalence or density of A. cantonensis L3 in catchment water.
It is commonly assumed that the severity of disease in a patient with NAS reflects the number of L3 that were ingested. In this regard, it bears remembering that even very small snails can carry high numbers of L3, sometimes in the thousands.
SIGNS AND SYMPTOMS, DIAGNOSIS AND TREATMENT
Infection with A. cantonensis is one of the leading causes of eosinophilic meningitis worldwide. After humans become infected, the most dramatic clinical findings reflect the presence of larvae in neurological tissues combined with a strong inflammatory reaction, especially as the larvae die. Occasionally, subadult worms as long as 1 to 3 cm appear in the eye, requiring surgical removal.45
Signs and symptoms are diverse, varying from patient to patient over days to weeks postinfection.46 There may be a prodromal phase as neurotropic L3 migrate from the intestinal tract, producing symptoms such as abdominal pain, nausea, vomiting, cough, dyspnea, headache, and a low-grade fever reflecting inflammation at multiple sites where the larvae lodge.47 When sought actively, early manifestations such as a rash (with or without pruritus), myalgias, and arthralgias probably occur in more than 20% of patients,48,49 sometimes within hours to days after infection (C. Panosian Dunavan, unpublished data).50,51 Although such symptoms are often overlooked or underreported, when sought actively and recognized, they can provide valuable clues enabling early presumptive diagnosis at a time when treatment with anthelminthics such as albendazole is particularly effective.47,52,53
Clinical features tend to be more specific after the parasites reach the central nervous system. Patients may experience migratory paresthesias and hyperesthesias in different parts of the body accompanied by myalgias often involving the neck and shoulders; severe, unremitting headaches; and bowel and bladder dysfunction resulting from radiculomyelitis.8 Occasionally, hydrocephalus, encephalitis, and cranial nerve palsies—even coma or death—later ensue. Some patients may suffer long-term disabilities with life-changing consequences; unfortunately, this form of the disease has been underappreciated for many years, especially in regions such as Australia and Hawaii, where the numbers of heavy infections seem to be rising.54,55
When NAS is suspected, a key diagnostic step is to perform a lumbar puncture to detect eosinophilia in the cerebrospinal fluid (CSF), and—in very rare cases—worms in the CSF, which confirms the diagnosis.46 However, because the signs and symptoms of NAS are diverse, delays in considering the diagnosis and performing lumbar punctures are common. This is true in both nonendemic and endemic regions, where few medical providers may be familiar with NAS. A valuable adjunct to diagnosis is the detection of A. cantonensis DNA in CSF via real-time polymerase chain reaction (PCR).56 More recent research has resulted in highly sensitive and ultrasensitive PCR tests that aim to detect very small amounts of A. cantonensis DNA not just in spinal fluid, but also in blood,57,58 possibly alleviating the need for lumbar puncture for diagnosis, although obtaining spinal fluid to exclude other diagnoses may still be necessary.
In some international laboratories, serological tests of blood and CSF have been used to detect acute infections resulting from A. cantonensis,59,60 but such tests are not available in the United States. In addition, because antibodies to A. cantonensis may take several weeks to develop after exposure to infective larvae, today, performing a DNA-based test is preferable in order to initiate treatment as early as possible. Last, in certain patients, empiric therapy may be justified based on epidemiological history and supportive clinical and laboratory findings including peripheral eosinophilia.
Following a diagnosis of NAS, many patients require analgesics for pain relief, and corticosteroids to lessen inflammation, sometimes together with repeat lumbar punctures to reduce intracranial pressure and relieve severe headaches. Additional options for pain relief include ketamine and intravenous lidocaine drugs.50,61,62 In challenging cases, a multidisciplinary approach to pain management is often necessary.
A final important therapy—anthelminthics to kill migrating worms—has been somewhat controversial, as some authors have speculated for decades that rapidly killing all the worms might produce a more damaging inflammatory reaction than would occur if subadult larvae died over a longer period of time.8,63 Nonetheless, a consensus has recently emerged that anthelminthics are useful, perhaps even key, especially if administered before or soon after the L3 reach the central nervous system, or at least before they molt and grow.46,47,64 It has also been suggested that anthelminthics be used prophylactically if, in an endemic area, a person realizes they may have been exposed to A. cantonensis, for instance by biting into a slug hidden in a sandwich; however, no study validating this approach is available.47
In short, avoiding the serious, sometimes devastating long-term consequences of severe infection should be a key objective for clinicians caring for patients with possible NAS. Early treatment with albendazole and corticosteroids, ideally taken within 2 weeks of infection, has been proposed as a way to limit chronic sequelae of the disease, despite the difficulty of diagnosing severely affected patients at such an early stage.47 Importantly, in a few cases, specific DNA in the CSF has been detected as early as 11 days after infection (V. Ansdell, unpublished data), and it would probably be detected even earlier in the CSF and blood using the previously mentioned highly sensitive or ultrasensitive PCR tests.57,58
GLOBAL CASES OF NEUROANGIOSTRONGYLIASIS
Angiostrongylus cantonensis is generally considered a tropical and subtropical parasite, limited by low temperatures. The territories with the highest numbers of reported cases of NAS are by far Thailand and China, where eating raw or undercooked snails is the primary route of human infection, with French Polynesia, where raw prawns (paratenic hosts) are the leading source of infection, a distant third.8 The United States is next with the vast majority of human infections contracted in Hawaii (see below), followed by Cuba, New Caledonia, and Japan.8 In Australia there were 28 known cases between 1971 and 2018, of which at least seven were travelers who had recently returned from Vanuatu and Fiji, where the disease is endemic; five of the 28 died.47 Elsewhere, very few human cases have been reported.
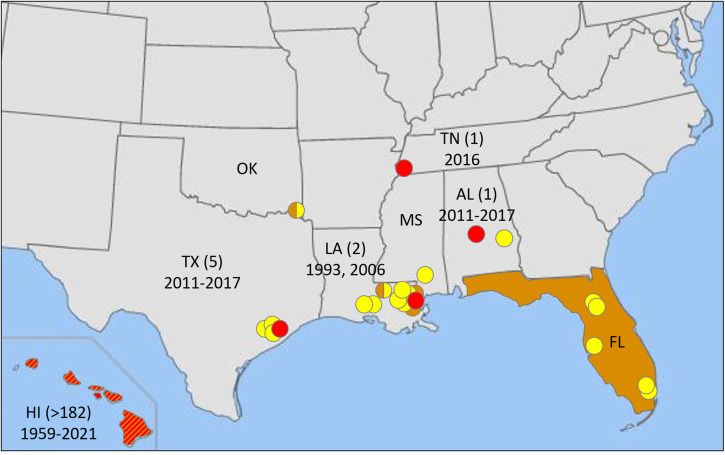
In the United States, A. cantonensis is broadly distributed throughout the Hawaiian Islands,65 where the great majority of human cases reported in the country have been contracted since the disease was first identified (Figure 3, Table 1). The parasite is also widely present across Florida, where it has been reported in nonhuman primates, an armadillo, and, in a statewide survey, in roughly 1 in 5 rats and 1 in 50 snails,66,75 yet surprisingly no human cases have been reported there. It is also present in Alabama, Louisiana, Oklahoma, Texas, and possibly Mississippi, based largely on reports in accidental hosts (Figure 3) including nonhuman primates, a horse, armadillos, and an opossum,66,67 with only a handful of autochthonous human cases reported in Texas, Louisiana, Alabama, and one as far north as Tennessee (Figure 3). A small number of additional infections in the continental United States have been detected in travelers returning from endemic parts of the world, including Hawaii (Figure 1).
Figure 3.
Known distribution of natural infections of hosts of Angiostrongylus cantonensis65–67 and autochthonous human cases of neuroangiostrongyliasis in the United States17,38,68: orange circles, A. cantonensis in definitive (rats) and intermediate (snails and slugs) hosts; yellow circles, A. cantonensis in nonhuman accidental hosts (see text for details); red circles, numbers of human cases by state and year. AL = Alabama, FL = Florida, HI = Hawaii, LA = Louisiana, MS = Mississippi, OK = Oklahoma, TN = Tennessee, TX = Texas. The parasite is widespread in Florida. In Hawaii (orange and red), there have been human cases on all six largest islands (Table 1), although A. cantonensis has not been detected on the island of Lanai.65 The record in Oklahoma was a rat (Sigmodon hispidus), and one record in Louisiana was also a rat (Neotoma sp.), but it is unclear whether either of these species was a definitive or an accidental host.69,70
Table 1.
Cases of neuroangiostrongyliasis in Hawaii, 1959 to 2021, by island
| Year | Island | ||||||
|---|---|---|---|---|---|---|---|
| Kauai | Oahu | Maui | Lanai | Hawaii | Unknown | Total | |
| 1959–1976 | 2 | 15 | 1 | 0 | 1 | 16 | 35 |
| 1977–1988 | 1 | 2 | 0 | 0 | 1 | 0 | 4 |
| 1989–2000 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| 2001–2004 | 0 | 12 | 3 | 1 | 4 | 0 | 20 |
| 2005 | 0 | 0 | 0 | 0 | 7 | 0 | 7 |
| 2006 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 2007 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| 2008 | 0 | 0 | 1 | 0 | 7 | 0 | 8 |
| 2009 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
| 2010 | 0 | 1 | 1 | 0 | 7 | 0 | 9 |
| 2011 | 0 | 0 | 0 | 0 | 7 | 0 | 7 |
| 2012 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 2013 | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| 2014 | 0 | 0 | 1 | 0 | 5 | 0 | 6 |
| 2015 | 1 | 0 | 0 | 0 | 6 | 0 | 7 |
| 2016 | 1 | 0 | 0 | 0 | 11 | 0 | 12 |
| 2017 | 0 | 1 | 8 | 0 | 13 | 0 | 22 |
| 2018 | 0 | 1 | 0 | 0 | 10 | 0 | 11 |
| 2019 | 1 | 0 | 0 | 0 | 8 | 0 | 9 |
| 2020 | 0 | 2 | 1 | 0 | 2 | 0 | 5 |
| 2021 | 0 | 1 | 1 | 0 | 3 | 0 | 5 |
| Total | 6 | 37 | 17 | 1 | 105 | 16 | 182 |
Despite the fact that A. cantonensis is the primary cause of eosinophilic meningitis worldwide, the total number of human infections recorded in the medical literature continues to be surprisingly low, currently ∼ 3,000,8 although including additional gray-literature records suggests the number is at least 7,000 (S. Lv, personal communication). However, many more cases have certainly gone unreported, either because symptoms were mild and short-lived and the infected person did not visit a doctor, or because the disease was misdiagnosed. Both of these possibilities are supported by a pilot seroepidemiological study conducted in Hawaii in 2015,76 in which 22% of 435 donated human blood specimens tested positive for antibodies to A. cantonensis when screened by crude-antigen ELISA and validated by a highly sensitive and specific 31-kDa dot-blot test originally developed in Thailand.
A lack of required reporting in endemic regions is another reason why global estimates of NAS may be woefully inaccurate. In Hawaii, NAS has been a reportable disease since 2007,77 and the state Department of Health maintains records of reported cases, with 1 to 22 cases reported per year since 2005 (Table 1). Prior to 2007, cases were identified and reported in the literature based on epidemiological surveys and case series reviews. Elsewhere, records are sketchy at best.
In addition to humans and wildlife, certain domestic and zoo animals can become infected as accidental hosts.66 Dogs are of particular interest because they are closely associated with humans and exhibit highly characteristic clinical signs suggesting NAS.78 As such, dogs, especially inquisitive, undiscerning puppies (just like some infants and toddlers) that eat or mouth an infected snail, could be considered sentinels for human NAS.79
AN EMERGING BUT NEGLECTED DISEASE
The range of A. cantonensis, an invasive species that has not yet reached every favorable locale, continues to expand. Many nonendemic parts of the world are susceptible; for instance, there are no records from most of Africa (Figure 1) and Europe is now threatened.14 Several studies of its potential range under climate warming suggest that A. cantonensis will extend or shift its range farther from the equator,69,80,81 including in the continental United States, where the parasite is likely to advance northward over time. In the Hawaiian Islands, its range will expand to include higher areas that are currently too cool for A. cantonensis.65
CONCLUSION
Both the medical community and the general public need to become more aware of neuroangiostrongyliasis—a rare but potentially fatal illness. Currently, most medical practitioners in nonendemic regions (and even in Hawaii, to some extent) possess little knowledge of rat lungworm disease. Increased awareness among the medical community will reduce the likelihood of sick people being misdiagnosed in primary care clinics and emergency rooms or simply given analgesics and anxiolytics, or of infected individuals waiting weeks to months before receiving an accurate diagnosis.68,82 In addition, increased awareness leading to prompt diagnosis and treatment will produce more favorable outcomes.46,47 As the parasite spreads, both travelers and residents of newly endemic regions will encounter it more frequently. Therefore, what may be most important of all is effective messaging that does not provoke fear, but allows at-risk residents and visitors to take appropriate precautions to avoid infection in the first place.
ACKNOWLEDGMENTS
This is University of Hawaii School of Ocean and Earth Sciences Publication No. 11580, and a publication of the Manoa Angiostrongylus Research Group.
REFERENCES
- 1.Beaver PC, Rosen L, 1964. Memorandum on the first report of Angiostrongylus in man, by Nomura and Lin, 1945. Am J Trop Med Hyg 13: 589–590. [DOI] [PubMed] [Google Scholar]
- 2.Kliks MM, Palumbo NE, 1992. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 34: 199–212. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BG, Little MD, 1988. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg 38: 568–573. [DOI] [PubMed] [Google Scholar]
- 4.Valente R, Robles M del R, Diaz JI, 2018. Gastropods as intermediate hosts of Angiostrongylus spp. in the Americas: bioecological characteristics and geographical distribution. Mem Inst Oswaldo Cruz 115: e200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C, 2014. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz 109: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alicata JE, Jindrak K, 1970. Angiostrongylosis in the Pacific and Southeast Asia. Springfield, IL: Charles C. Thomas. [Google Scholar]
- 7.Bisseru B, 1971. The prevalence of Angiostrongylus cantonensis larvae collected from giant African snail, Achatina fulica in West Malaysia and Singapore. Southeast Asian J Trop Med Public Health 2: 523–526. [PubMed] [Google Scholar]
- 8.Wang Q-P, Lai D-H, Zhu X-Q, Chen X-G, Lun Z-R, 2008. Human angiostrongyliasis. Lancet Infect Dis 8: 621–630. [DOI] [PubMed] [Google Scholar]
- 9.Cowie RH, 2013. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health 72 (Suppl 2): 6–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J, Stark D, 2016. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143: 1087–1118. [DOI] [PubMed] [Google Scholar]
- 11.Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Sadaow L, Laymanivong S, Aung WP, Phosuk I, Laummaunwai P, Maleewong W, 2016. Angiostrongylus cantonensis and A. malaysiensis broadly overlap in Thailand, Lao PDR, Cambodia and Myanmar: a molecular survey of larvae in land snails. PLoS One 11: e0161128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansdell V, Wattanagoon Y, 2018. Angiostrongylus cantonensis in travelers: clinical manifestations, diagnosis, and treatment. Curr Opin Infect Dis 31: 399–408. [DOI] [PubMed] [Google Scholar]
- 13.Defo AL. et al. , 2018. Angiostrongylus cantonensis infection of central nervous system, Guiana Shield. Emerg Infect Dis 24: 1153–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado-Serra S, Sola J, Negre N, Paredes-Esquivel C, 2022. Angiostrongylus cantonensis nematode invasion pathway, Mallorca, Spain. Emerg Infect Dis 28: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dard C, Tessier E, Nguyen D, Epelboin L, Harrois D, Swale C, Cabié A, de Meuron K, Miossec C, Desbois-Nogard M, 2020. First cases of Angiostrongylus cantonensis infection reported in Martinique, 2002–2017. Parasite 27: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federspiel F, Skovmand S, Skarphedinsson S, 2020. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int J Infect Dis 93: 28–39. [DOI] [PubMed] [Google Scholar]
- 17.Liu EW. et al. , 2018. Rat lungworm infection associated with central nervous system disease – eight U.S. states, January 2011–January 2017. MMWR Morb Mortal Wkly Rep 67: 825–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noskin GA, McMenamin MB, Grohmann SM, 1992. Eosinophilic meningitis due to _Angiostrongylus cantonensis._Neurology 42: 1423–1424. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Carrillo N. et al. , 2021. A peculiar distribution of the emerging nematode Angiostrongylus cantonensis in the Canary Islands (Spain): recent introduction or isolation effect? Animals (Basel) 11: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yong HS, Eamsobhana P, 2013. Definitive rodent hosts of the rat lungworm _Angiostrongylus cantonensis._Raffles Bull Zool Suppl 29: 111–115. [Google Scholar]
- 21.Kim JR, Hayes KA, Yeung NW, Cowie RH, 2014. Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian Islands. PLoS One 9: e94969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turck HC, Fox MT, Cowie RH, 2022. Paratenic hosts of Angiostrongylus cantonensis and their relation to human neuroangiostrongyliasis globally. One Health 15: 100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New D, Little MD, Cross J, 1995. Angiostrongylus cantonensis infection from eating raw snails. N Engl J Med 332: 1105–1106. [DOI] [PubMed] [Google Scholar]
- 24.Fischer PR, 1983. Eosinophilic meningitis. West J Med 139: 372–373. [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon E, Ferguson TM, Park SY, Manuzak A, Qvarnstrom Y, Morgan S, Ciminera P, Murphy GS, 2013. A severe case of Angiostrongylus eosinophilic meningitis with encephalitis and neurologic sequelae in Hawaiʻi. Hawaii J Med Public Health 72 (Suppl 2): 41–45. [PMC free article] [PubMed] [Google Scholar]
- 26.Widder JR, Fallah S, Mondzelewski TJ, 2020. A case report of slug ingestion causing eosinophilic meningitis, papilledema, and pronounced motor weakness in a US marine. Mil Med 185: 317–321. [DOI] [PubMed] [Google Scholar]
- 27.Alicata JE, 1963. The incidence of Angiostrongylus cantonensis (Chen) and mollusks in New Caledonia and nearby islands and its possible relationship to eosinophilic meningitis. South Pacific Commission Technical Paper 139. 1–9.
- 28.Senanayake SN, Pryor DS, Walker J, Konecny P, 2003. First report of human angiostrongyliasis acquired in Sydney. Med J Aust 179: 430–431. [DOI] [PubMed] [Google Scholar]
- 29.Blair NF, Orr CF, Delanay AP, Herkes GK, 2010. Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation. Med J Aust 198: 440–442. [DOI] [PubMed] [Google Scholar]
- 30.Lv S, Zhang Y, Chen S-R, Wang L-B, Fang W, Chen F, Jiang J-Y, Li Y-L, Du Z-W, Zhou X-N, 2009. Human angiostrongyliasis outbreak in Dali, China. PLoS Negl Trop Dis 3: e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eamsobhana P, Yoolek A, Yong H-S, 2010. Effect of Thai ‘koi-hoi’ food flavoring on the viability and infectivity of the third-stage larvae of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae). Acta Trop 113: 245–247. [DOI] [PubMed] [Google Scholar]
- 32.Cowie RH, 2013. Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawaii J Med Public Health 72 (Suppl 2): 70–74. [PMC free article] [PubMed] [Google Scholar]
- 33.Nalini A, Ramakrishna A, Dekumoy P, Kumar RR, Pakdee W, Saini J, Hegde VS, 2013. Severe form of radiculo-myelo-neuropathy with meningo-encephalitis secondary to Angiostrongylus cantonensis infection: unusual corpus callosal lesions and serial magnetic resonance imaging findings. Neurol India 61: 414–418. [DOI] [PubMed] [Google Scholar]
- 34.Pai S, Madi D, Achappa B, Mahalingam S, Kendambadi R, 2013. An interesting case of eosinophilic meningitis. J Clin Diagn Res 7: 734–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Darasavath C, Chang K, Vilay V, Sengduangphachanh A, Adsamouth A, Vongsouvath M, Keolouangkhot V, Robinson MT, 2021. Cluster of angiostrongyliasis cases following consumption of raw monitor lizard in the Lao People’s Democratic Republic and review of the literature. Trop Med Infect Dis 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Lu L, She D, Wen Z, Mo Z, Li J, Li H, 2018. Eating centipedes can result in Angiostrongylus cantonensis infection: two case reports and pathogen investigation. Am J Trop Med Hyg 99: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai H-C, Lai P-H, Sy C-L, Lee SS-J, Yen C-M, Wann S-R, Chen Y-S, 2011. Encephalitis caused by Angiostrongylus cantonensis after eating raw frogs mixed with wine as a health supplement. Intern Med 50: 771–774. [DOI] [PubMed] [Google Scholar]
- 38.Cuneo P, Clement S, Sokol T, 2006. Eosinophilic meningitis and _Angiostrongylus cantonensis._Louisiana Morbidity Rep 17: 1. [Google Scholar]
- 39.Tsai H-C, Lee SS-J, Huang C-K, Yen C-M, Chen E-R, Liu Y-C, 2004. Outbreak of eosinophilic meningitis associated with drinking raw vegetable juice in southern Taiwan. Am J Trop Med Hyg 71: 222–226. [PubMed] [Google Scholar]
- 40.Slom TJ. et al. , 2002. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346: 668–675. [DOI] [PubMed] [Google Scholar]
- 41.Ash LR, 1976. Observations on the role of mollusks and planarians in the transmission of Angiostrongylus cantonensis infection to man in New Caledonia. Rev Biol Trop 24: 163–174. [Google Scholar]
- 42.Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K, Kawanaka M, 2004. Changing epidemiology of Angiostrongylus cantonensis in Okinawa Prefecture, Japan. Jpn J Infect Dis 57: 184–186. [PubMed] [Google Scholar]
- 43.Kramer KJ, Posner J, Gosnell WL, 2018. Role of gastropod mucus in the transmission of Angiostrongylus cantonensis, a potentially serious neurological infection. ACS Chem Neurosci 9: 629–632. [DOI] [PubMed] [Google Scholar]
- 44.Howe K, Kaluna L, Lozano A, Torres Fischer B, Tagami Y, McHugh R, Jarvi S, 2019. Water transmission potential of Angiostrongylus cantonensis: larval viability and effectiveness of rainwater catchment sediment filters. PLoS One 14: e0209813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Y, Nawa Y, Sawanyawisuth K, Lv Z, Wu Z-D, 2013. Comprehensive review of ocular angiostrongyliasis with special reference to optic neuritis. Korean J Parasitol 51: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansdell V. et al. , 2021. Guidelines for the diagnosis and treatment of neuroangiostrongyliasis: updated recommendations. Parasitology 148: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berkhout A, Prociv P, Anthony H, Anthony LT, Clare N, 2019. Two cases of neuroangiostrongyliasis: a rare disease because rarely considered or rarely diagnosed? J Paediatr Child Health 55: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 48.Tsai H-C. et al. , 2001. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 111: 109–114. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg NS, Blackburn BG, Park SY, Sejvar JJ, Effler PV, Herwaldt BL, 2011. Eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in Hawaii: clinical characteristics and potential exposures. Am J Trop Med Hyg 85: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busse J, Gottlieb D, Ferreras K, Bain J, Schechter W, 2018. Pharmacological management of severe neuropathic pain in a case of eosinophilic meningitis related to _Angiostrongylus cantonensis._Case Rep Anesthesiol 2018: Article ID 5038272, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAuliffe L. et al. , 2019. Severe CNS angiostrongyliasis in a young marine: a case report and literature review. Lancet Infect Dis 19: e132–e142. [DOI] [PubMed] [Google Scholar]
- 52.Hwang K-P, Chen E-R, 1988. Larvicidal effect of albendazole against A. cantonensis in mice. Am J Trop Med Hyg 39: 191–195. [DOI] [PubMed] [Google Scholar]
- 53.Prociv P, Turner M, 2018. Neuroangiostrongyliasis: the “Subarachnoid Phase” and its implications for anthelminthic therapy. Am J Trop Med Hyg 98: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid IR, Wallis WE, 1984. The chronic and severe forms of eosinophilic meningitis. Aust N Z J Med 14: 163–165. [DOI] [PubMed] [Google Scholar]
- 55.Meyer BC, 2021. Chronic neuroangiostrongyliasis: case study of chronic presentations in Hawaii. Parasitology 148: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qvarnstrom Y, 2016. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am J Trop Med Hyg 94: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sears WJ. et al. , 2021. AcanR3990 qPCR: a novel, highly sensitive, bioinformatically-informed assay to detect Angiostrongylus cantonensis infections. Clin Infect Dis 73: e1594–e1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sears WJ, Qvarnstrom Y, Nutman TB, 2021. RPAcan3990: an ultrasensitive recombinase polymerase assay to detect Angiostrongylus cantonensis DNA. J Clin Microbiol 59: e01185-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eamsobhana P, Prasartvit A, Gan XX, Yong HS, 2015. Evaluation of dot immunogold filtration assay (DIGFA) for rapid serodiagnosis of eosinophilic meningitis due to Angiostrongylus cantonensis (Nematoda: Metastrongyloidea). Trop Biomed 32: 121–125. [PubMed] [Google Scholar]
- 60.Morassutti AL, Rascoe LN, Handali S, Da Silva AJ, Wilkins PP, Graeff-Teixeira C, 2017. Cross-reactivity of the 31 kDa antigen of Angiostrongylus cantonensis – dealing with the immunodiagnosis of meningoencephalitis. Parasitology 144: 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cucueco K, Bathen C, Fischberg D, 2020. Lidocaine infusion for refractory pain from rat lungworm disease – Honolulu, Hawai’i. Hawaii J Health Soc Welf 79: 246–248. [PMC free article] [PubMed] [Google Scholar]
- 62.Yates J, Devere T, Sakurai-Burton S, Santi B, McAllister C, Frank K, 2022. Angiostrongylus cantonensis infection presenting as small fiber neuropathy. Am J Trop Med Hyg 107: 367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pien FD, Pien BC, 1999. Angiostrongylus cantonensis eosinophilic meningitis. Int J Infect Dis 3: 161–163. [DOI] [PubMed] [Google Scholar]
- 64.Jacob J, Tan G, Lange I, Saeed H, Date A, Jarvi S, 2021. In vitro efficacy of anthelmintics on Angiostrongylus cantonensis L3 larvae. Parasitology 148: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JR, Wong TW, Curry PA, Yeung NW, Hayes KA, Cowie RH, 2019. Modelling the distribution in Hawaii of Angiostrongylus cantonensis (rat lungworm) in its gastropod hosts. Parasitology 146: 42–49. [DOI] [PubMed] [Google Scholar]
- 66.Walden HDS, Slapcinsky J, Rosenberg J, Wellehan JFX, 2021. Angiostrongylus cantonensis (rat lungworm) in Florida, USA: current status. Parasitology 148: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizor J, Yanez RA, Thaiwong T, Kiupel M, 2022. Angiostrongylus cantonensis in a red ruffed lemur at a zoo, Louisiana, USA. Emerg Infect Dis 28: 1058–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flerlage T, Qvarnstrom Y, Noh J, Devincenzo JP, Madni A, Bagga B, Hysmith ND, 2017. Angiostrongylus cantonensis eosinophilic meningitis in an infant, Tennessee, USA. Emerg Infect Dis 23: 1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.York EM, Creecy JP, Lord WD, Caire W, 2015. Geographic range expansion for the rat lungworm in North America. Emerg Infect Dis 21: 1234–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim DY, Stewart TB, Bauer RW, Mitchell M, 2002. Parastrongylus (= Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol 88: 1024–1026. [DOI] [PubMed] [Google Scholar]
- 71.Cowie RH, 2017. Angiostrongylus cantonensis: agent of a sometimes fatal globally emerging infectious disease (rat lungworm disease). ACS Chem Neurosci 8: 2102–2104. [DOI] [PubMed] [Google Scholar]
- 72.Kuberski T, Wallace GD, 1979. Clinical manifestations of eosinophilic meningitis due to _Angiostrongylus cantonensis._Neurology 29: 1566–1570. [DOI] [PubMed] [Google Scholar]
- 73.Koo J, Pien F, Kliks MM, 1988. Angiostrongylus (Parastrongylus) eosinophilic meningitis. Rev Infect Dis 10: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 74.Hughes PA, Magnet AD, Fishbain JT, 2003. Eosinophilic meningitis: a case series report and review of the literature. Mil Med 168: 817–821. [PubMed] [Google Scholar]
- 75.Walden HDS, Slapcinsky JD, Roff S, Mendieta Calle JM, Diaz Goodwin Z, Stern J, Corlett R, Conway J, McIntosh A, 2017. Geographic distribution of Angiostrongylus cantonensis in wild rats (Rattus rattus) and terrestrial snails in Florida, USA. PLoS One 12: e0177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarvi SI. et al. , 2020. Estimating human exposure to rat lungworm (Angiostrongylus cantonensis) on Hawai’i Island: a pilot study. Am J Trop Med Hyg 102: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnston DI, Dixon MC, Elm JL, Jr., Calimlim PS, Sciulli RH, Park SY, 2019. Review of cases of angiostrongyliasis in Hawaii, 2007–2017. Am J Trop Med Hyg 101: 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Odani J, Sox E, Coleman W, Jha R, Malik R, 2021. First documented cases of canine neuroangiostrongyliasis due to Angiostrongylus cantonensis in Hawaii. J Am Anim Hosp Assoc 57: 42–46. [DOI] [PubMed] [Google Scholar]
- 79.Lunn JA, Lee R, Smaller J, MacKay BM, King T, Hunt GB, Martin P, Krockenberger MB, Spielman D, Malik R, 2012. Twenty two cases of canine neural angiostronglyosis in eastern Australia (2002–2005) and a review of the literature. Parasit Vectors 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lv S, Zhang Y, Steinmann P, Yang G-J, Yang K, Zhou X-N, Utzinger J, 2011. The emergence of angiostrongyliasis in the People’s Republic of China: the interplay between invasive snails, climate change and transmission dynamics. Freshw Biol 56: 717–734. [Google Scholar]
- 81.York EM, Butler CJ, Lord WD, 2014. Global decline in suitable habitat for Angiostrongylus (= Parastrongylus) cantonensis: the role of climate change. PLoS One 9: e103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howe K, 2013. A severe case of rat lungworm disease in Hawaiʻi. Hawaii J Med Public Health 72 (Suppl 2): 46–48. [PMC free article] [PubMed] [Google Scholar]