The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions - PubMed (original) (raw)
The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions
D R Sheff et al. J Cell Biol. 1999.
Abstract
Receptor recycling involves two endosome populations, peripheral early endosomes and perinuclear recycling endosomes. In polarized epithelial cells, either or both populations must be able to sort apical from basolateral proteins, returning each to its appropriate plasma membrane domain. However, neither the roles of early versus recycling endosomes in polarity nor their relationship to each other has been quantitatively evaluated. Using a combined morphological, biochemical, and kinetic approach, we found these two endosome populations to represent physically and functionally distinct compartments. Early and recycling endosomes were resolved on Optiprep gradients and shown to be differentially associated with rab4, rab11, and transferrin receptor; rab4 was enriched on early endosomes and at least partially depleted from recycling endosomes, with the opposite being true for rab11 and transferrin receptor. The two populations were also pharmacologically distinct, with AlF4 selectively blocking export of transferrin receptor from recycling endosomes to the basolateral plasma membrane. We applied these observations to a detailed kinetic analysis of transferrin and dimeric IgA recycling and transcytosis. The data from these experiments permitted the construction of a testable, mathematical model which enabled a dissection of the roles of early and recycling endosomes in polarized receptor transport. Contrary to expectations, the majority (>65%) of recycling to the basolateral surface is likely to occur from early endosomes, but with relatively little sorting of apical from basolateral proteins. Instead, more complete segregation of basolateral receptors from receptors intended for transcytosis occurred upon delivery to recycling endosomes.
Figures
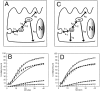
Figure 6
The kinetics of Tfn recycling in MDCK cells is consistent with the existence of two distinct endosomal recycling compartments. (A) A kinetic model of endocytosis assuming a single pathway through two consecutive internal compartments. First order rate constants (k n) for passage of ligand along a given pathway segment are shown. k n represents anterograde transport, while k −n represents retrograde transport. (B) Best fit of the single pathway model was optimized by minimizing the value of the sum squared variances between predicted and experimental values. Data points (open square, control recycled; open triangle, control transcytosed; closed square, AlF4 recycled; closed triangle, AlF4 transcytosed) represent the actual experimental data to which curve fitting was attempted. Solid lines were derived from the mathematical model (black lines are recycled ligand, gray transcytosed ligand, solid lines are control cells, dashed are AlF4 treated). Note the relatively poor fit of the model to recycled Tfn in AlF4-treated cells (SS = 334 untreated, SS = 836 treated). (C) Addition of rate constants describing potential pathways from EEs direct to the basolateral plasma membrane (k 4) or the apical plasma membrane (k 6). (D) Fitting of the modified model described in C to experimentally determined data points. As in B, symbols represent the data and lines are generated from the modified model. The degree of fit was far better (Fischer's F-test of comparative fit: F = 48 for control, 424 for AlF4; P < 0.05 for both, see text) than in B (SS = 140 untreated, SS = 64 AlF4-treated).
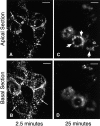
Figure 1
FITC-Tfn passes sequentially through peripheral and perinuclear endosomes in polarized MDCK cells. MDCK cells were transfected with the human TfnR and grown on clear Transwell filters. FITC-Tfn was bound to the basolateral surface at 0°C and internalized at 37°C for 2.5 min (A and B) or 25 min (C and D). Fluorescence images were obtained with focal planes at 4.5 μm (A and C) and 0.5 μm (B and D) from the filter surface. After 2.5 min, FITC-Tfn was localized in punctate basolateral structures in the basal and apical cytoplasm (A and B, arrows). By 25 min, the FITC-Tfn shifted to larger, more apical and perinuclear structures (C, arrows). Bar, 10 μm.
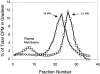
Figure 2
Separation of EEs and REs by Optiprep gradient centrifugation. MDCK cells transfected with the human TfnR were grown on Transwell filters. 125I-Tfn was bound at 0°C and then internalized for either 2.5 or 25 min. The cells were acid washed to remove remaining surface bound 125I-Tfn, homogenized, and postnuclear supernatants separated on 5–20% linear Optiprep gradients. The low density region of the gradients is on the left (fraction 1). 125I-Tfn internalized for 2.5 min was contained within membranes which sedimented towards the bottom of the gradient (squares). After 25 min of chase, the Tfn was recovered as a single peak of slightly lower density (circles). The position of the plasma membrane was determined by alkaline phosphodiesterase activity and used to indicate the position of plasma membranes on the gradient. β-Hexosaminidase activity (lysosomes) formed a characteristically well defined peak in fractions 9–15 (not shown). The radioactivity in each fraction was normalized to percent total label loaded in each gradient (191,589 cpm for 2.5-min time point; 40,767 cpm for 25-min time point).
Figure 3
EEs and REs have distinct protein compositions. MDCK cells cotransfected with human TfnR and pIgR were grown on Transwell filters and crude postnuclear supernatants (including cytosol) were centrifuged using 10–20% Optiprep density gradients. (A) 125I-Tfn was bound at 0°C and then internalized for 2.5 or 25 min as in Fig. 2. Postnuclear supernatants were prepared and then centrifuged. Percent total radioactivity in each gradient is indicated for the 2.5-min time point (squares) and the 25-min time point (triangles). 125I-Tfn peaks around fraction 5 cosediment with the basolateral plasma membrane marker alkaline phosphatase; more 125I-Tfn was found in this region than in Fig. 2 because cells were not extensively acid washed before homogenization. EEs are distinguished by a peak at fraction 23 and REs by a peak at fraction 20. (B) Western blots of fractionated cells using antibodies to human TfnR, human rab4, and human rab11. Half of the entire volume of each fraction was loaded in each lane. TfnR was found in all fractions but is most abundant in the RE-containing fractions identified by the 25-min 125I-Tfn peak. rab4 cosedimented most closely with the EE peak; rab11 was relatively depleted from these EE-containing fractions relative to higher density fractions more coincident with REs (TfnR, 25 min 125I-Tfn). (C) Double label immunoelectron microscopy of endosomes isolated on Optiprep density gradients. Tfn receptor was labeled with 5-nm gold in both images (small arrowheads). Fraction 24 was additionally labeled for rab11 with 10-nm gold (large arrowheads). Fraction 27 was labeled for rab4 with 10-nm gold (large arrowheads).
Figure 3
EEs and REs have distinct protein compositions. MDCK cells cotransfected with human TfnR and pIgR were grown on Transwell filters and crude postnuclear supernatants (including cytosol) were centrifuged using 10–20% Optiprep density gradients. (A) 125I-Tfn was bound at 0°C and then internalized for 2.5 or 25 min as in Fig. 2. Postnuclear supernatants were prepared and then centrifuged. Percent total radioactivity in each gradient is indicated for the 2.5-min time point (squares) and the 25-min time point (triangles). 125I-Tfn peaks around fraction 5 cosediment with the basolateral plasma membrane marker alkaline phosphatase; more 125I-Tfn was found in this region than in Fig. 2 because cells were not extensively acid washed before homogenization. EEs are distinguished by a peak at fraction 23 and REs by a peak at fraction 20. (B) Western blots of fractionated cells using antibodies to human TfnR, human rab4, and human rab11. Half of the entire volume of each fraction was loaded in each lane. TfnR was found in all fractions but is most abundant in the RE-containing fractions identified by the 25-min 125I-Tfn peak. rab4 cosedimented most closely with the EE peak; rab11 was relatively depleted from these EE-containing fractions relative to higher density fractions more coincident with REs (TfnR, 25 min 125I-Tfn). (C) Double label immunoelectron microscopy of endosomes isolated on Optiprep density gradients. Tfn receptor was labeled with 5-nm gold in both images (small arrowheads). Fraction 24 was additionally labeled for rab11 with 10-nm gold (large arrowheads). Fraction 27 was labeled for rab4 with 10-nm gold (large arrowheads).
Figure 3
EEs and REs have distinct protein compositions. MDCK cells cotransfected with human TfnR and pIgR were grown on Transwell filters and crude postnuclear supernatants (including cytosol) were centrifuged using 10–20% Optiprep density gradients. (A) 125I-Tfn was bound at 0°C and then internalized for 2.5 or 25 min as in Fig. 2. Postnuclear supernatants were prepared and then centrifuged. Percent total radioactivity in each gradient is indicated for the 2.5-min time point (squares) and the 25-min time point (triangles). 125I-Tfn peaks around fraction 5 cosediment with the basolateral plasma membrane marker alkaline phosphatase; more 125I-Tfn was found in this region than in Fig. 2 because cells were not extensively acid washed before homogenization. EEs are distinguished by a peak at fraction 23 and REs by a peak at fraction 20. (B) Western blots of fractionated cells using antibodies to human TfnR, human rab4, and human rab11. Half of the entire volume of each fraction was loaded in each lane. TfnR was found in all fractions but is most abundant in the RE-containing fractions identified by the 25-min 125I-Tfn peak. rab4 cosedimented most closely with the EE peak; rab11 was relatively depleted from these EE-containing fractions relative to higher density fractions more coincident with REs (TfnR, 25 min 125I-Tfn). (C) Double label immunoelectron microscopy of endosomes isolated on Optiprep density gradients. Tfn receptor was labeled with 5-nm gold in both images (small arrowheads). Fraction 24 was additionally labeled for rab11 with 10-nm gold (large arrowheads). Fraction 27 was labeled for rab4 with 10-nm gold (large arrowheads).
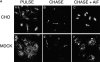
Figure 4
Partial retention of FITC-Tfn in REs by AlF4. (A) CHO cells and MDCK cells expressing human TfnR were pulsed with FITC-Tfn for 30 min at 37°C, conditions which labeled both peripheral and perinuclear endosome populations (panels A and B). The cells were then chased in the presence of excess unlabeled Tfn for 30 min (panels C and D). Although a few cells remained brightly labeled, most exhibited a marked loss of FITC-Tfn, with remaining cell-associated FITC-Tfn found predominantly in perinuclear vesicles (arrows). When cells were chased in the presence of unlabeled Tfn and AlF4 for 30 min, however, FITC-Tfn was largely retained in the perinuclear endosomes (panels E and F). In CHO cells, these structures exhibited the characteristic distribution as a tightly organized array near the microtubule organizing center; in MDCK cells, they were more diffusely distributed, but nevertheless closely apposed to the nucleus. (B) X-Z reconstructions from confocal fluorescence imaging of intact, fully polarized monolayers of MDCK cells transfected with the human TfnR. Cells were loaded with FITC-Tfn for 20 min at 37°C (top). The cells were chased in the presence of unlabeled Tfn for 40 min in the absence (middle) or presence (bottom) of AlF4. Arrows indicate the apically located FITC-Tfn–containing endosomes; gray lines indicate the position of the Transwell filters and thus the basolateral surface of the cell monolayer. Each panel has been contrast processed with Adobe Photoshop to emphasize the position of the label rather than the total amount. (C) Optiprep gradient centrifugation of MDCK cells after 125I-Tfn uptake for 2.5 min (squares) or 25 min (circles) in the presence (closed symbols) or absence (open symbols) of AlF4. Low density fractions are shown to the left.
Figure 4
Partial retention of FITC-Tfn in REs by AlF4. (A) CHO cells and MDCK cells expressing human TfnR were pulsed with FITC-Tfn for 30 min at 37°C, conditions which labeled both peripheral and perinuclear endosome populations (panels A and B). The cells were then chased in the presence of excess unlabeled Tfn for 30 min (panels C and D). Although a few cells remained brightly labeled, most exhibited a marked loss of FITC-Tfn, with remaining cell-associated FITC-Tfn found predominantly in perinuclear vesicles (arrows). When cells were chased in the presence of unlabeled Tfn and AlF4 for 30 min, however, FITC-Tfn was largely retained in the perinuclear endosomes (panels E and F). In CHO cells, these structures exhibited the characteristic distribution as a tightly organized array near the microtubule organizing center; in MDCK cells, they were more diffusely distributed, but nevertheless closely apposed to the nucleus. (B) X-Z reconstructions from confocal fluorescence imaging of intact, fully polarized monolayers of MDCK cells transfected with the human TfnR. Cells were loaded with FITC-Tfn for 20 min at 37°C (top). The cells were chased in the presence of unlabeled Tfn for 40 min in the absence (middle) or presence (bottom) of AlF4. Arrows indicate the apically located FITC-Tfn–containing endosomes; gray lines indicate the position of the Transwell filters and thus the basolateral surface of the cell monolayer. Each panel has been contrast processed with Adobe Photoshop to emphasize the position of the label rather than the total amount. (C) Optiprep gradient centrifugation of MDCK cells after 125I-Tfn uptake for 2.5 min (squares) or 25 min (circles) in the presence (closed symbols) or absence (open symbols) of AlF4. Low density fractions are shown to the left.
Figure 4
Partial retention of FITC-Tfn in REs by AlF4. (A) CHO cells and MDCK cells expressing human TfnR were pulsed with FITC-Tfn for 30 min at 37°C, conditions which labeled both peripheral and perinuclear endosome populations (panels A and B). The cells were then chased in the presence of excess unlabeled Tfn for 30 min (panels C and D). Although a few cells remained brightly labeled, most exhibited a marked loss of FITC-Tfn, with remaining cell-associated FITC-Tfn found predominantly in perinuclear vesicles (arrows). When cells were chased in the presence of unlabeled Tfn and AlF4 for 30 min, however, FITC-Tfn was largely retained in the perinuclear endosomes (panels E and F). In CHO cells, these structures exhibited the characteristic distribution as a tightly organized array near the microtubule organizing center; in MDCK cells, they were more diffusely distributed, but nevertheless closely apposed to the nucleus. (B) X-Z reconstructions from confocal fluorescence imaging of intact, fully polarized monolayers of MDCK cells transfected with the human TfnR. Cells were loaded with FITC-Tfn for 20 min at 37°C (top). The cells were chased in the presence of unlabeled Tfn for 40 min in the absence (middle) or presence (bottom) of AlF4. Arrows indicate the apically located FITC-Tfn–containing endosomes; gray lines indicate the position of the Transwell filters and thus the basolateral surface of the cell monolayer. Each panel has been contrast processed with Adobe Photoshop to emphasize the position of the label rather than the total amount. (C) Optiprep gradient centrifugation of MDCK cells after 125I-Tfn uptake for 2.5 min (squares) or 25 min (circles) in the presence (closed symbols) or absence (open symbols) of AlF4. Low density fractions are shown to the left.
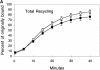
Figure 5
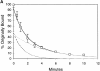
AlF4 causes missorting during Tfn recycling in MDCK cells. (A) MDCK cells transfected with the human TfnR were grown on Transwell filters. 125I-Tfn was bound to the basolateral membrane on ice. Cells were warmed to 37°C to initiate endocytosis. Appearance of total label in both the apical and basal media was combined and shown in the presence (closed squares) or absence (open squares) of AlF4 (n = 3 for each condition). (B) Experiment as in A except that media was collected separately from the upper chamber (transcytosis, triangles, n = 2) or lower chamber (recycling, squares, n = 4 for AlF4, n = 3 for control) in the absence (open symbols) or presence (closed symbols) of AlF4. Error bars represent standard deviation when n > 2 or standard error when n = 2.
Figure 5
AlF4 causes missorting during Tfn recycling in MDCK cells. (A) MDCK cells transfected with the human TfnR were grown on Transwell filters. 125I-Tfn was bound to the basolateral membrane on ice. Cells were warmed to 37°C to initiate endocytosis. Appearance of total label in both the apical and basal media was combined and shown in the presence (closed squares) or absence (open squares) of AlF4 (n = 3 for each condition). (B) Experiment as in A except that media was collected separately from the upper chamber (transcytosis, triangles, n = 2) or lower chamber (recycling, squares, n = 4 for AlF4, n = 3 for control) in the absence (open symbols) or presence (closed symbols) of AlF4. Error bars represent standard deviation when n > 2 or standard error when n = 2.
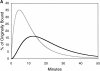
Figure 7
Predicted kinetics of appearance of Tfn in EEs and REs. (A) Using the mathematical model described in Fig. 6, C and D, the predicted amounts of Tfn in the EEs (gray line) and REs (black line) are shown over the course of the experiment in untreated cells. (B) Predicted Tfn amounts of Tfn in EEs and REs as in A but now in the presence of AlF4. EE (gray line) and RE (black line) are shown. Note the increased duration of Tfn in REs as compared with controls (A).
Figure 7
Predicted kinetics of appearance of Tfn in EEs and REs. (A) Using the mathematical model described in Fig. 6, C and D, the predicted amounts of Tfn in the EEs (gray line) and REs (black line) are shown over the course of the experiment in untreated cells. (B) Predicted Tfn amounts of Tfn in EEs and REs as in A but now in the presence of AlF4. EE (gray line) and RE (black line) are shown. Note the increased duration of Tfn in REs as compared with controls (A).
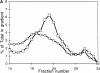
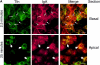
Figure 8
Tfn and dIgA transit through the same EE and RE populations. (A) FITC-Tfn and Texas red–dIgA were bound to the basolateral surface of MDCK cells doubly transfected with the TfnR and pIgR at 0°C. The ligands were then internalized at 37°C for 2.5 (top) or 25 min (bottom). Tfn (green) and dIgA (red) were imaged separately and merged; yellow spots indicate likely double-positive structures. 2.5-min images were taken 0.5 μm from the filter surface; 25-min images were taken ∼5 μm from the filter surface, in the apical cytoplasm. Bar, 10 μm. (B) 125I-Tfn or 125I-dIgA were bound to the basolateral surface of filter-grown MDCK cells doubly transfected with TfnR and pIgR at 0°C. The ligands were internalized at 37°C for 2.5 min, then the cells were harvested and homogenates were fractionated in parallel on Optiprep density gradients. EE peak is at fraction 29. (C) After 25 min of internalization, both Tfn and dIgA shifted to apparently the same, less dense RE compartment. Closed symbols indicate Tfn, open symbols indicate dIgA.
Figure 8
Tfn and dIgA transit through the same EE and RE populations. (A) FITC-Tfn and Texas red–dIgA were bound to the basolateral surface of MDCK cells doubly transfected with the TfnR and pIgR at 0°C. The ligands were then internalized at 37°C for 2.5 (top) or 25 min (bottom). Tfn (green) and dIgA (red) were imaged separately and merged; yellow spots indicate likely double-positive structures. 2.5-min images were taken 0.5 μm from the filter surface; 25-min images were taken ∼5 μm from the filter surface, in the apical cytoplasm. Bar, 10 μm. (B) 125I-Tfn or 125I-dIgA were bound to the basolateral surface of filter-grown MDCK cells doubly transfected with TfnR and pIgR at 0°C. The ligands were internalized at 37°C for 2.5 min, then the cells were harvested and homogenates were fractionated in parallel on Optiprep density gradients. EE peak is at fraction 29. (C) After 25 min of internalization, both Tfn and dIgA shifted to apparently the same, less dense RE compartment. Closed symbols indicate Tfn, open symbols indicate dIgA.
Figure 8
Tfn and dIgA transit through the same EE and RE populations. (A) FITC-Tfn and Texas red–dIgA were bound to the basolateral surface of MDCK cells doubly transfected with the TfnR and pIgR at 0°C. The ligands were then internalized at 37°C for 2.5 (top) or 25 min (bottom). Tfn (green) and dIgA (red) were imaged separately and merged; yellow spots indicate likely double-positive structures. 2.5-min images were taken 0.5 μm from the filter surface; 25-min images were taken ∼5 μm from the filter surface, in the apical cytoplasm. Bar, 10 μm. (B) 125I-Tfn or 125I-dIgA were bound to the basolateral surface of filter-grown MDCK cells doubly transfected with TfnR and pIgR at 0°C. The ligands were internalized at 37°C for 2.5 min, then the cells were harvested and homogenates were fractionated in parallel on Optiprep density gradients. EE peak is at fraction 29. (C) After 25 min of internalization, both Tfn and dIgA shifted to apparently the same, less dense RE compartment. Closed symbols indicate Tfn, open symbols indicate dIgA.
Figure 9
Kinetic analysis of dimeric IgA internalization. (A) Dissecting the contribution of rapid dIgA dissociation from internalization. MDCK cells transfected with the rabbit pIgR cDNA were grown on Transwell filters. 125I-dIgA was bound to the basolateral surface on ice. The cells were warmed to 37°C, and at 2-min intervals individual filters were removed to ice-cold buffer and surface IgA stripped with a pH 2.9 wash. Disappearance of the acid sensitive counts (squares, n = 3) is a direct measure of dIgA clearance from the basolateral surface. Mathematically derived curves for dissociation (dashed line) and internalization (dotted line) were added together to get the mathematically predicted rate of surface clearance (solid line) which is compared with the experimentally derived values (in this model internalization and dissociation are from distinct pools of dIgA, SS = 104, see text). All counts were normalized to a percentage of counts initially bound to each Transwell filter. Error bars represent standard deviation. (B) The kinetics of dIgA recycling into the basolateral medium for early time points was plotted after correcting for the rapid dissociation of dIgA from pIgR before internalization (A). Open squares represent individual data points indicating the percentage of radioactivity initially bound. Line represents results of a mathematical model including both direct fall-off from the basolateral membrane into the basolateral media and recycling of internalized ligand from the EE to the basolateral media. The same kinetic rate constants used in A are used in B (SS = 63).
Figure 9
Kinetic analysis of dimeric IgA internalization. (A) Dissecting the contribution of rapid dIgA dissociation from internalization. MDCK cells transfected with the rabbit pIgR cDNA were grown on Transwell filters. 125I-dIgA was bound to the basolateral surface on ice. The cells were warmed to 37°C, and at 2-min intervals individual filters were removed to ice-cold buffer and surface IgA stripped with a pH 2.9 wash. Disappearance of the acid sensitive counts (squares, n = 3) is a direct measure of dIgA clearance from the basolateral surface. Mathematically derived curves for dissociation (dashed line) and internalization (dotted line) were added together to get the mathematically predicted rate of surface clearance (solid line) which is compared with the experimentally derived values (in this model internalization and dissociation are from distinct pools of dIgA, SS = 104, see text). All counts were normalized to a percentage of counts initially bound to each Transwell filter. Error bars represent standard deviation. (B) The kinetics of dIgA recycling into the basolateral medium for early time points was plotted after correcting for the rapid dissociation of dIgA from pIgR before internalization (A). Open squares represent individual data points indicating the percentage of radioactivity initially bound. Line represents results of a mathematical model including both direct fall-off from the basolateral membrane into the basolateral media and recycling of internalized ligand from the EE to the basolateral media. The same kinetic rate constants used in A are used in B (SS = 63).
Figure 10
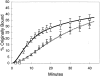
The two-compartment, two-pathway model developed for Tfn accounts for observed kinetics of IgA recycling and transcytosis. Corrected experimental data points for basolateral recycling (squares) and apical transcytosis (triangles) are shown and compared with best fit curves calculated using the model described in Fig. 6 C. Curves resulting from modeling of the data fit closely, suggesting that this model is consistent with the data (SS = 20, F = 83 compared with a single pathway).
Figure 11
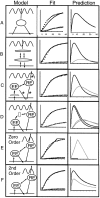
Alternative mathematical models are not all equal. Alternative mathematical models were constructed to describe endosomal processing of Tfn. Representative alternatives are illustrated in column 1 (Model). To be acceptable they must fit the experimental kinetic data (column 2, Fit) and make accurate predictions about the passage of Tfn through EEs and REs. For each model, the predicted curves for Tfn localization in EE and RE at various time points are shown in column 3 (Prediction). For all fit graphs, squares are recycling data, triangles are transcytosis data, black line is modeled recycling, and gray line is modeled transcytosis; y-axis is 0–100% of initially bound Tfn, x-axis is 0–40 min. For all prediction graphs, black line is EE and gray line is RE; y-axis is 0–40% of initially bound Tfn, x-axis is 0–40 min. (A) A single compartment model in the presence of AlF4 fails to fit the recycling data (column 2, Fit) or to account for the observed passage of Tfn through the two physically distinct endosome populations. (B) A dual compartment model in which there is no direct pathway from the RE to the basolateral surface incorrectly predicts that the amount of Tfn in the EE will be greater than or equal to that in the RE in untreated cells even at 25 min after internalization (see column 3, Prediction). (C) Addition of a compartment between the basolateral membrane and the EE still fits the kinetic data and makes an acceptable prediction about internal localization as long as the rate of surface clearance into the new compartment is 0.21 (equal to the calculated rate from basolateral membrane to EE in our model) and the rate out of the new compartment to the EE is >1. Additional compartments may also be placed at asterisks with similar results (not shown). (D) Addition of a compartment between EEs and REs under AlF4 conditions fits kinetic experimental data but incorrectly predicts little change in the amount of Tfn in RE after addition of AlF4. In the prediction column, the two black lines indicate Tfn in EE, and the two gray lines indicate Tfn in RE. The upper line at 20 min in each pair represents the AlF4-treated cells. (E) A two-compartment, two-pathway model as described in Fig. 6 C but using zero order rate constants fails to match the form of the progress curves generated experimentally. (F) A two-compartment, two-pathway model as described in Fig. 6 C but using second order rate constants fits the experimentally derived data, but incorrectly predicts that for untreated cells, the level of Tfn in EE will always exceed that in REs.
Similar articles
- Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes.
Sheff DR, Kroschewski R, Mellman I. Sheff DR, et al. Mol Biol Cell. 2002 Jan;13(1):262-75. doi: 10.1091/mbc.01-07-0320. Mol Biol Cell. 2002. PMID: 11809838 Free PMC article. - Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome.
Wang E, Brown PS, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Wang E, et al. Traffic. 2000 Jun;1(6):480-93. doi: 10.1034/j.1600-0854.2000.010606.x. Traffic. 2000. PMID: 11208134 - Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells.
Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Casanova JE, et al. Mol Biol Cell. 1999 Jan;10(1):47-61. doi: 10.1091/mbc.10.1.47. Mol Biol Cell. 1999. PMID: 9880326 Free PMC article. - Regulation of membrane transport through the endocytic pathway by rabGTPases.
Mohrmann K, van der Sluijs P. Mohrmann K, et al. Mol Membr Biol. 1999 Jan-Mar;16(1):81-7. doi: 10.1080/096876899294797. Mol Membr Biol. 1999. PMID: 10332741 Review. - Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity.
Jing J, Prekeris R. Jing J, et al. Histol Histopathol. 2009 Sep;24(9):1171-80. doi: 10.14670/HH-24.1171. Histol Histopathol. 2009. PMID: 19609864 Free PMC article. Review.
Cited by
- GLUT4 exocytosis.
Stöckli J, Fazakerley DJ, James DE. Stöckli J, et al. J Cell Sci. 2011 Dec 15;124(Pt 24):4147-59. doi: 10.1242/jcs.097063. J Cell Sci. 2011. PMID: 22247191 Free PMC article. Review. - Differential compartmentalization of BMP4/NOGGIN requires NOGGIN trans-epithelial transport.
Phan-Everson T, Etoc F, Li S, Khodursky S, Yoney A, Brivanlou AH, Siggia ED. Phan-Everson T, et al. Dev Cell. 2021 Jul 12;56(13):1930-1944.e5. doi: 10.1016/j.devcel.2021.05.003. Epub 2021 May 28. Dev Cell. 2021. PMID: 34051144 Free PMC article. - Major Determinants of Airway Epithelial Cell Sensitivity to S. aureus Alpha-Toxin: Disposal of Toxin Heptamers by Extracellular Vesicle Formation and Lysosomal Degradation.
Möller N, Ziesemer S, Hentschker C, Völker U, Hildebrandt JP. Möller N, et al. Toxins (Basel). 2021 Feb 24;13(3):173. doi: 10.3390/toxins13030173. Toxins (Basel). 2021. PMID: 33668237 Free PMC article. - Protein-Decorated Microbubbles for Ultrasound-Mediated Cell Surface Manipulation.
Brans VA, Gray MD, Sezgin E, Stride EPJ. Brans VA, et al. ACS Appl Bio Mater. 2023 Dec 18;6(12):5746-5758. doi: 10.1021/acsabm.3c00861. Epub 2023 Dec 4. ACS Appl Bio Mater. 2023. PMID: 38048163 Free PMC article. - Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment.
Strick DJ, Elferink LA. Strick DJ, et al. Mol Biol Cell. 2005 Dec;16(12):5699-709. doi: 10.1091/mbc.e05-03-0204. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195351 Free PMC article.
References
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous