An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation - PubMed (original) (raw)
An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation
C Asseman et al. J Exp Med. 1999.
Abstract
A T helper cell type 1-mediated colitis develops in severe combined immunodeficient mice after transfer of CD45RB(high) CD4(+) T cells and can be prevented by cotransfer of the CD45RB(low) subset. The immune-suppressive activities of the CD45RB(low) T cell population can be reversed in vivo by administration of an anti-transforming growth factor beta antibody. Here we show that interleukin (IL)-10 is an essential mediator of the regulatory functions of the CD45RB(low) population. This population isolated from IL-10-deficient (IL-10(-/-)) mice was unable to protect from colitis and when transferred alone to immune-deficient recipients induced colitis. Treatment with an anti-murine IL-10 receptor monoclonal antibody abrogated inhibition of colitis mediated by wild-type (WT) CD45RB(low) CD4(+) cells, suggesting that IL-10 was necessary for the effector function of the regulatory T cell population. Inhibition of colitis by WT regulatory T cells was not dependent on IL-10 production by progeny of the CD45RB(high) CD4(+) cells, as CD45RB(low) CD4(+) cells from WT mice were able to inhibit colitis induced by IL-10(-/-) CD45RB(high) CD4(+) cells. These findings provide the first clear evidence that IL-10 plays a nonredundant role in the functioning of regulatory T cells that control inflammatory responses towards intestinal antigens.
Figures
Figure 1
Representative photomicrographs of the descending colon of RAG-2−/− mice after transfer of subpopulations of CD4+ T cells from WT or IL-10−/− mice. (A) Severe colitis in a mouse injected with CD45RBhigh CD4+ T cells from WT mice. (B) Normal appearance of the colon in a mouse restored with WT CD45RBhigh and WT CD45RBlow CD4+ T cells, indicating that the WT CD45RBlow population is able to inhibit disease induced by WT CD45RBhigh CD4+ T cells. (C) Severe colitis in a mouse cotransferred with WT CD45RBhigh CD4+ T cells and IL-10−/− CD45RBlow CD4+ cells, indicating that the IL-10−/− CD45RBlow subpopulation is unable to protect from the disease. (D) Severe colitis in a mouse receiving only IL-10−/− CD45RBlow cells, indicating that this population is able to induce disease. Hematoxylin and eosin; original magnifications: ×50.
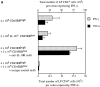
Figure 2
IFN-γ and TNF-α production by LP CD4+ T cells from colons. RAG-2−/− or C.B-17 SCID recipients were reconstituted with cell subsets as indicated. 8–12 wk after cell reconstitution, LP cells were isolated and stimulated for 12 h with anti-CD3∈ antibody. Levels of cytokine expression were determined by cytofluorography. (A) Absolute number of CD4+ cytokine-positive cells per colon. Numbers were determined by multiplying the frequency of cytokine-secreting CD4+ T cells by the total number of CD4+ cells. Data represent the mean ± SEM of two to five animals per group. (B) Frequency of cytokine-secreting CD4+ T cells. Data are gated on CD4+ T cells and are representative examples for each group. Horizontal lines represent staining with isotype control mAb.
Figure 2
IFN-γ and TNF-α production by LP CD4+ T cells from colons. RAG-2−/− or C.B-17 SCID recipients were reconstituted with cell subsets as indicated. 8–12 wk after cell reconstitution, LP cells were isolated and stimulated for 12 h with anti-CD3∈ antibody. Levels of cytokine expression were determined by cytofluorography. (A) Absolute number of CD4+ cytokine-positive cells per colon. Numbers were determined by multiplying the frequency of cytokine-secreting CD4+ T cells by the total number of CD4+ cells. Data represent the mean ± SEM of two to five animals per group. (B) Frequency of cytokine-secreting CD4+ T cells. Data are gated on CD4+ T cells and are representative examples for each group. Horizontal lines represent staining with isotype control mAb.
Similar articles
- A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells.
Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. Powrie F, et al. J Exp Med. 1996 Jun 1;183(6):2669-74. doi: 10.1084/jem.183.6.2669. J Exp Med. 1996. PMID: 8676088 Free PMC article. - Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10.
Asseman C, Read S, Powrie F. Asseman C, et al. J Immunol. 2003 Jul 15;171(2):971-8. doi: 10.4049/jimmunol.171.2.971. J Immunol. 2003. PMID: 12847269 - B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis.
Liu Z, Geboes K, Hellings P, Maerten P, Heremans H, Vandenberghe P, Boon L, van Kooten P, Rutgeerts P, Ceuppens JL. Liu Z, et al. J Immunol. 2001 Aug 1;167(3):1830-8. doi: 10.4049/jimmunol.167.3.1830. J Immunol. 2001. PMID: 11466409 - Control of experimental inflammatory bowel disease by regulatory T cells.
Asseman C, Fowler S, Powrie F. Asseman C, et al. Am J Respir Crit Care Med. 2000 Oct;162(4 Pt 2):S185-9. doi: 10.1164/ajrccm.162.supplement_3.15tac9. Am J Respir Crit Care Med. 2000. PMID: 11029392 Review. - Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation.
Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Strober W, et al. Immunol Today. 1997 Feb;18(2):61-4. doi: 10.1016/s0167-5699(97)01000-1. Immunol Today. 1997. PMID: 9057354 Review.
Cited by
- The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review.
Stepkowski S, Bekbolsynov D, Oenick J, Brar S, Mierzejewska B, Rees MA, Ekwenna O. Stepkowski S, et al. Vaccines (Basel). 2024 Aug 30;12(9):992. doi: 10.3390/vaccines12090992. Vaccines (Basel). 2024. PMID: 39340024 Free PMC article. Review. - Posttransplant complications: molecular mechanisms and therapeutic interventions.
Liu X, Shen J, Yan H, Hu J, Liao G, Liu D, Zhou S, Zhang J, Liao J, Guo Z, Li Y, Yang S, Li S, Chen H, Guo Y, Li M, Fan L, Li L, Luo P, Zhao M, Liu Y. Liu X, et al. MedComm (2020). 2024 Sep 2;5(9):e669. doi: 10.1002/mco2.669. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39224537 Free PMC article. Review. - Distinct characteristics of unique immunoregulatory canine non-conventional TCRαβpos CD4negCD8αneg double-negative T cell subpopulations.
Karwig L, Moore PF, Alber G, Eschke M. Karwig L, et al. Front Immunol. 2024 Aug 9;15:1439213. doi: 10.3389/fimmu.2024.1439213. eCollection 2024. Front Immunol. 2024. PMID: 39185407 Free PMC article. - A chemogenetic screen reveals that Trpv1-expressing neurons control regulatory T cells in the gut.
Zhu Y, Meerschaert KA, Galvan-Pena S, Bin NR, Yang D, Basu H, Kawamoto R, Shalaby A, Liberles SD, Mathis D, Benoist C, Chiu IM. Zhu Y, et al. Science. 2024 Aug 2;385(6708):eadk1679. doi: 10.1126/science.adk1679. Epub 2024 Aug 2. Science. 2024. PMID: 39088603 Free PMC article. - Protective function of sclerosing cholangitis on IBD.
Bedke T, Stumme F, Tomczak M, Steglich B, Jia R, Bohmann S, Wittek A, Kempski J, Göke E, Böttcher M, Reher D, Franke A, Lennartz M, Clauditz T, Sauter G, Fründt T, Weidemann S, Tiegs G, Schramm C, Gagliani N, Pelczar P, Huber S. Bedke T, et al. Gut. 2024 Jul 11;73(8):1292-1301. doi: 10.1136/gutjnl-2023-330856. Gut. 2024. PMID: 38839272 Free PMC article.
References
- Elson C.O., Sartor R.B., Tennyson G.S., Riddell R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. - PubMed
- Powrie F., Leach M.W. Genetic and spontaneous models of inflammatory bowel disease in rodentsevidence for abnormalities in mucosal immune regulation. Ther. Immunol. 1995;2:115–123. - PubMed
- Morrissey P.J., Charrier K., Braddy S., Liggitt D., Watson J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178:237–244. - PMC - PubMed
- Powrie F., Leach M.W., Mauze S., Caddle L.B., Coffman R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 scid mice. Int. Immunol. 1993;5:1461–1471. - PubMed
- Powrie F., Leach M.W., Mauze S., Menon S., Caddle L.B., Coffman R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials