Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension - PubMed (original) (raw)
Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension
T Wallerath et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Hypertension is a side effect of systemically administered glucocorticoids, but the underlying molecular mechanism remains poorly understood. Ingestion of dexamethasone by rats telemetrically instrumented increased blood pressure progressively over 7 days. Plasma concentrations of Na(+) and K(+) and urinary Na(+) and K(+) excretion remained constant, excluding a mineralocorticoid-mediated mechanism. Plasma NO(2)(-)/NO(3)(-) (the oxidation products of NO) decreased to 40%, and the expression of endothelial NO synthase (NOS III) was found down-regulated in the aorta and several other tissues of glucocorticoid-treated rats. The vasodilator response of resistance arterioles was tested by intravital microscopy in the mouse dorsal skinfold chamber model. Dexamethasone treatment significantly attenuated the relaxation to the endothelium-dependent vasodilator acetylcholine, but not to the endothelium-independent vasodilator S-nitroso-N-acetyl-D,L-penicillamine. Incubation of human umbilical vein endothelial cells, EA.hy 926 cells, or bovine aortic endothelial cells with several glucocorticoids reduced NOS III mRNA and protein expression to 60-70% of control, an effect that was prevented by the glucocorticoid receptor antagonist mifepristone. Glucocorticoids decreased NOS III mRNA stability and reduced the activity of the human NOS III promoter (3.5 kilobases) to approximately 70% by decreasing the binding activity of the essential transcription factor GATA. The expressional down-regulation of endothelial NOS III may contribute to the hypertension caused by glucocorticoids.
Figures
Figure 1
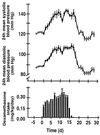
Effect of dexamethasone in the drinking water (3 mg/liter) on systolic and diastolic blood pressure of rats (Upper). Dexamethasone intake was monitored continuously with a PC-controlled monitoring system. The exact dose of dexamethasone ingested by the rats in 24 h is shown in the lower panel. Systolic and diastolic blood pressures were measured telemetrically before (○), during (●), and after (○) dexamethasone treatment. Values and bars represent means ± SEM obtained from five rats.
Figure 2
Effect of treatment of male rats with dexamethasone on NOS III mRNA expression in different tissues. Rats remained untreated [control (Co)] or were treated for 3, 6, and 9 days with dexamethasone (3 mg/liter in the drinking water, resulting in an approximate dose of 0.3 mg/kg/day). The dexamethasone treatment induced a significant decrease in NOS III mRNA expression in aorta (■) and liver (●) and a moderate decrease in kidney (□). The figure shows densitometric analyses of five independent experiments. Bars represent means ± SEM. Asterisks indicate significant differences from untreated rats (*, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Figure 3
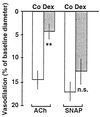
Dexamethasone treatment (0.3 mg/kg/day) significantly attenuated endothelium-dependent relaxation of resistance arterioles as assessed by intravital microscopy in awake C57/BL 6J mice. Shown is the vasorelaxation of resistance arterioles as percent of baseline microvessel diameter. The mean baseline diameters were 37.9 ± 6.5 μm in control animals (Co, white bars) and 45.0 ± 9.8 μm in dexamethasone-treated animals (Dex, gray bars). Depicted in the figure are changes in the responses to acetylcholine (ACh, 10 μM) and SNAP (10 μM). Data represent means ± SEM of n = 7 animals per group. Asterisks indicate significant differences from control animals (**, P < 0.01, Wilcoxon rank sum test) n.s., not significant.
Figure 4
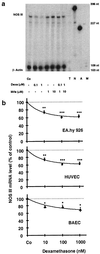
Down-regulation of NOS III mRNA by dexamethasone and reversal of the effect by the glucocorticoid receptor antagonist mifepristone (Mife) in endothelial cells. a shows an autoradiograph of a representative gel of an RNase protection experiment. RNase protection analyses were performed by using antisense RNA probes to human NOS III and β-actin (for standardization) and RNA from EA.hy 926 cells incubated with 0.1 or 1 μM dexamethasone, 1 or 10 μM mifepristone, or a combination of both. T, tRNA control; N, undigested NOS III antisense probe; A, undigested β-actin antisense probe; M, molecular size marker (pGl2-Basic, restricted with _Hin_fI). The gel is representative of three similar experiments. b shows the down-regulation of NOS III mRNA in three types of endothelial cells: EA.hy 926 cells (●), human umbilical vein endothelial cells (HUVEC, ■), and bovine aortic endothelial cells (BAEC, ▴, using bovine antisense RNA probes). The cells either were left untreated [control cells (Co)] or were incubated with dexamethasone (10 nM to 1 μM) for 18 h. Values represent means ± SEM. Asterisks indicate significant differences from untreated cells (*, P < 0.05; **, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Figure 5
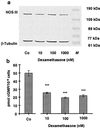
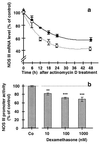
Effect of an incubation of EA.hy 926 cells with dexamethasone for 36 h on NOS III protein expression and NOS activity. a shows a Western blot analysis using a polyclonal anti-NOS III- and a monoclonal anti-β-tubulin-antibody (for normalization). Combined cytosolic and solubilized particulate protein fractions were prepared from untreated EA.hy 926 cells [control (Co)] and EA.hy 926 cells incubated with dexamethasone (10–1,000 nmol) for 36 h. M, molecular weight marker. The blot shown is representative of four independent experiments with similar results. b shows the effect of a 36-h incubation with dexamethasone on the NO production of EA.hy 926 cells stimulated for 2 min with Ca2+ ionophore A23187 (10 μM). NO production was measured by the activation of soluble guanylyl cyclase in RFL-6 reporter cells. cGMP content of the RFL-6 cells after a 2-min incubation was determined by radioimmunoassay. Basal cGMP content of the RFL-6 cells (2.12 ± 0.4 pmol/106 cells) was subtracted from all samples. Data represent means ± SEM of three independent experiments. Asterisks indicate significant differences from untreated cells (***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Figure 6
Effect of dexamethasone on NOS III mRNA stability and NOS III promoter activity in EA.hy 926 cells. In a, EA.hy 926 cells were preincubated without (●) or with 100 nM dexamethasone (□) for 18 h. Then, the inhibitor of transcription actinomycin D (10 μg/ml) was added to the culture medium. RNA was prepared 0, 12, 18, 24, and 48 h thereafter, and NOS III mRNA was determined by RNase protection assays. The NOS III mRNA levels in both groups were set at 100% at the time of addition of actinomycin D (0 h). Values are means ± SEM of three independent experiments. b shows EA.hy 926 cells stably transfected with pNOS III-3500-Hu-Luc-neo (containing a 3.5-kilobase NOS III promoter fragment cloned before a luciferase reporter gene). Stable cells either were kept untreated [control (Co)] or were incubated with dexamethasone (10–1,000 nM) for 24 h. Then, the cells were lysed, and luciferase activity was determined. The luciferase activity was taken as a measure of NOS III promoter activity. Bars represent means ± SEM of three independent experiments. Asterisks indicate significant differences from untreated cells (**, P < 0.01; ***, P < 0.001 by ANOVA and Fisher’s PLSD test).
Figure 7
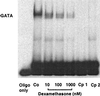
Electrophoretic mobility shift assay showing the effect of dexamethasone on the binding activity of nuclear extracts from EA.hy 926 cells to a double-stranded oligonucleotide containing the binding motif for transcription factor GATA of the human NOS III promoter (positions −239 to −218; 5′-GCTCCCACTTATCAGCCTCAGT-3′). Nuclear extracts were prepared from control cells (Co) or from cells incubated for 18 h with dexamethasone (10 nM to 1 μM) and were incubated with the radiolabeled oligonucleotide. Samples were analyzed on native polyacrylamide gels. Lane 1 shows radiolabeled oligonucleotide alone (oligo only). Lanes 5 and 6 (Cp1 and Cp2) show competition experiments with extracts from control (Co) cells and the addition of 100-fold excess of unlabeled oligonucleotide 5′-CACTTACAGAAAGTGATAACTCT-3′ (containing a consensus GATA binding motif), or unlabeled oligonucleotide 5′-GCTCCCACTTATCAGCCTCAGT-3′ (containing the GATA site of the human NOS III promoter), respectively. The gel shown is representative of four gels yielding the same results.
Similar articles
- Dexamethasone suppresses eNOS and CAT-1 and induces oxidative stress in mouse resistance arterioles.
Schäfer SC, Wallerath T, Closs EI, Schmidt C, Schwarz PM, Förstermann U, Lehr HA. Schäfer SC, et al. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H436-44. doi: 10.1152/ajpheart.00587.2004. Am J Physiol Heart Circ Physiol. 2005. PMID: 15598872 - Sodium nitrite causes relaxation of the isolated rat aorta: By stimulating both endothelial NO synthase and activating soluble guanylyl cyclase in vascular smooth muscle.
Ling WC, Lau YS, Murugan DD, Vanhoutte PM, Mustafa MR. Ling WC, et al. Vascul Pharmacol. 2015 Nov;74:87-92. doi: 10.1016/j.vph.2015.05.014. Epub 2015 Jun 2. Vascul Pharmacol. 2015. PMID: 26044183 - Angiotensin-converting enzyme inhibition and angiotensin AT1-receptor antagonism equally improve endothelial vasodilator function in L-NAME-induced hypertensive rats.
De Gennaro Colonna V, Rigamonti A, Fioretti S, Bonomo S, Manfredi B, Ferrario P, Bianchi M, Berti F, Muller EE, Rossoni G. De Gennaro Colonna V, et al. Eur J Pharmacol. 2005 Jun 15;516(3):253-9. doi: 10.1016/j.ejphar.2005.04.004. Eur J Pharmacol. 2005. PMID: 15963975 - The nitric oxide system in glucocorticoid-induced hypertension.
Whitworth JA, Schyvens CG, Zhang Y, Andrews MC, Mangos GJ, Kelly JJ. Whitworth JA, et al. J Hypertens. 2002 Jun;20(6):1035-43. doi: 10.1097/00004872-200206000-00003. J Hypertens. 2002. PMID: 12023661 Review. - Glucocorticoid-induced hypertension.
Goodwin JE, Geller DS. Goodwin JE, et al. Pediatr Nephrol. 2012 Jul;27(7):1059-66. doi: 10.1007/s00467-011-1928-4. Epub 2011 Jul 9. Pediatr Nephrol. 2012. PMID: 21744056 Review.
Cited by
- Health position paper and redox perspectives - Disease burden by transportation noise.
Sørensen M, Pershagen G, Thacher JD, Lanki T, Wicki B, Röösli M, Vienneau D, Cantuaria ML, Schmidt JH, Aasvang GM, Al-Kindi S, Osborne MT, Wenzel P, Sastre J, Fleming I, Schulz R, Hahad O, Kuntic M, Zielonka J, Sies H, Grune T, Frenis K, Münzel T, Daiber A. Sørensen M, et al. Redox Biol. 2024 Feb;69:102995. doi: 10.1016/j.redox.2023.102995. Epub 2023 Dec 18. Redox Biol. 2024. PMID: 38142584 Free PMC article. Review. - Metabolomic Profiling of Hormonal Contraceptive Use in Young Females Using a Commercially Available LC-MS/MS Kit.
Grobler T, Opperman M, Bester J, Swanepoel AC, du Preez I. Grobler T, et al. Metabolites. 2023 Oct 18;13(10):1092. doi: 10.3390/metabo13101092. Metabolites. 2023. PMID: 37887417 Free PMC article. - Long-Term Exposure to Traffic Noise and Risk of Incident Cardiovascular Diseases: a Systematic Review and Dose-Response Meta-Analysis.
Fu X, Wang L, Yuan L, Hu H, Li T, Zhang J, Ke Y, Wang M, Gao Y, Huo W, Chen Y, Zhang W, Liu J, Huang Z, Zhao Y, Hu F, Zhang M, Liu Y, Sun X, Hu D. Fu X, et al. J Urban Health. 2023 Aug;100(4):788-801. doi: 10.1007/s11524-023-00769-0. Epub 2023 Aug 14. J Urban Health. 2023. PMID: 37580544 Free PMC article. Review. - Endothelial Cell Response in Kawasaki Disease and Multisystem Inflammatory Syndrome in Children.
Kim J, Shimizu C, He M, Wang H, Hoffman HM, Tremoulet AH, Shyy JY, Burns JC. Kim J, et al. Int J Mol Sci. 2023 Aug 1;24(15):12318. doi: 10.3390/ijms241512318. Int J Mol Sci. 2023. PMID: 37569694 Free PMC article. - Living Alone Increases the Risk of Hypertension in Older Chinese Adults: A Population-Based Longitudinal Study.
Wang X, Yuan X, Xia B, He Q, Jie W, Dai M. Wang X, et al. Innov Aging. 2023 Jul 3;7(6):igad071. doi: 10.1093/geroni/igad071. eCollection 2023. Innov Aging. 2023. PMID: 37502337 Free PMC article.
References
- Huang P L, Huang Z H, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. - PubMed
- Alheid U, Frölich J C, Förstermann U. Thromb Res. 1987;47:561–571. - PubMed
- Radomski M W, Palmer R M, Moncada S. Biochem Biophys Res Commun. 1987;148:1482–1489. - PubMed
- Arndt H, Smith C W, Granger D N. Hypertension. 1993;21:667–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical