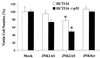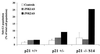Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner - PubMed (original) (raw)
Inhibition of c-Jun N-terminal kinase 2 expression suppresses growth and induces apoptosis of human tumor cells in a p53-dependent manner
O Potapova et al. Mol Cell Biol. 2000 Mar.
Abstract
c-Jun N-terminal kinase (JNK) plays a critical role in coordinating the cellular response to stress and has been implicated in regulating cell growth and transformation. To investigate the growth-regulatory functions of JNK1 and JNK2, we used specific antisense oligonucleotides (AS) to inhibit their expression. A survey of several human tumor cell lines revealed that JNKAS treatment markedly inhibited the growth of cells with mutant p53 status but not that of cells with normal p53 function. To further examine the influence of p53 on cell sensitivity to JNKAS treatment, we compared the responsiveness of RKO, MCF-7, and HCT116 cells with normal p53 function to that of RKO E6, MCF-7 E6, and HCT116 p53(-/-), which were rendered p53 deficient by different methods. Inhibition of JNK2 (and to a lesser extent JNK1) expression dramatically reduced the growth of p53-deficient cells but not that of their normal counterparts. JNK2AS-induced growth inhibition was correlated with significant apoptosis. JNK2AS treatment induced the expression of the cyclin-dependent kinase inhibitor p21(Cip1/Waf1) in parental MCF-7, RKO, and HCT116 cells but not in the p53-deficient derivatives. That p21(Cip1/Waf1) expression contributes to the survival of JNK2AS-treated cells was supported by additional experiments demonstrating that p21(Cip1/Waf1) deficiency in HCT116 cells also results in heightened sensitivity to JNKAS treatment. Our results indicate that perturbation of JNK2 expression adversely affects the growth of otherwise nonstressed cells. p53 and its downstream effector p21(Cip1/Waf1) are important in counteracting these detrimental effects and promoting cell survival.
Figures
FIG. 1
MCF-7 and RKO cells display high-efficiency uptake of oligonucleotides delivered via transfection with Lipofectin reagent. Cells were treated with 0.3 μM 3′ FITC-labeled control oligonucleotide as described in Materials and Methods. Twenty-four hours later, they were fixed and examined for fluorescence with a confocal microscope. Images were obtained at a magnification of ×800. (Left) Confocal fluorescent images of transfected cells. (Right) Confocal transparent images of the same fields. Virtually all cells show uptake of the oligonucleotides, irrespective of p53 status.
FIG. 2
JNKAS treatment effectively inhibits JNK expression in MCF-7 and RKO cells regardless of p53 status. (A) Total RNA was extracted from cells 24 h following treatment with 0.3 μM JNK1AS, JNK2AS, or control oligonucleotides (JNKScr). RNA samples were analyzed by Northern analysis using cDNA probes specific for JNK1 and JNK2 mRNAs. (B) Whole-cell lysates were examined by Western analysis for JNK expression 24 h following treatment with JNKAS oligonucleotides. (C) Total Jun kinase activity was determined 30 min following exposure to UVC (40 J/m2) by an in vitro kinase assay using GST-cJun(1-222) as a substrate. At 24 h prior to UVC treatment, cells were transfected with combinations of JNK1AS plus JNK2AS (0.15 μM each) or JNK1Scr plus JNK2Scr (0.15 μM each) (labeled JNKAS and JNKScr, respectively).
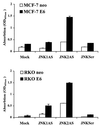
FIG. 3
Effect of JNKAS treatment on the viability of p53-proficient and p53-deficient MCF-7 and RKO cells. Cells were seeded in 96-well cluster plates and treated with 0.3 μM oligonucleotides in Lipofectin reagent (JNKScr designates treatment with 0.15 μM JNK1Scr plus 0.15 μM JNK2Scr). Cell viability was assessed at the indicated times by measuring MTS dye in a colorimetric assay as described in Materials and Methods.
FIG. 4
Morphological appearance of JNK2AS-treated MCF-7 and RKO cells with normal (neo) or deficient (E6) p53 function. Cells treated with 0.3 μM of the indicated oligonucleotides were examined by optical microscopy 24 h after lipofection. Cultures that were subjected to either mock lipofection or treatment with scrambled oligonucleotides (the same as described in the legend to Fig. 3) had morphologies similar to those seen in untreated control cultures. The density of JNKAS-treated E6-expressing MCF-7 and RKO cells is lower than that of the neo-expressing counterparts. JNK2AS-treated p53-deficient (E6-expressing) cells exhibit membrane blebbing and an increase in the number of rounded and/or detached cells.
FIG. 5
Biochemical evidence of DNA degradation in p53-deficient cells treated with JNKAS. Apoptosis of p53-proficient (neo) and p53-deficient (E6) MCF-7 and RKO cells was assessed 24 h after treatment with 0.3 μM JNKAS or control oligonucleotides using an ELISA that measures nucleosomal degradation. JNKScr depicts results for treatments with equal amounts of JNK1Scr plus JNK2Scr (0.15 μM each). Results are expressed as relative absorption (OD) at 405 nm.
FIG. 6
FACS analysis of mock-transfected and JNKAS-treated RKO cells. RKO neo and RKO E6-expressing cells were treated with either JNK1AS, JNK2AS, or a combination of control scrambled oligonucleotides (JNKScr). They were harvested 24 h following the lipofection, and 2 × 106 cells were subjected to DNA content analysis by FACS. The percentage of total cells contained in the sub-G1 peak of JNK2AS and JNK1AS- plus JNK2AS-treated RKO E6 cell cultures is indicated in the figure (arrow). For all other treatments of E6-expressing RKO and RKO neo cells, the sub-G1 fraction was 1.5% or less.
FIG. 7
Inhibition of JNK expression in HCT116 cells results in growth suppression in a p53-dependent manner. Viable cell mass was measured at 24 h, 48 h (shown here), and 72 h following treatment with JNKAS and control oligonucleotides as described in Materials and Methods. Growth inhibition following JNK2AS treatment is p53 dependent: ∗, statistically significant difference between the number of viable cells in JNK2AS-treated cultures of parental HCT116 cells and HCT116 p53−/− cells (P < 0.003, Student's t test).
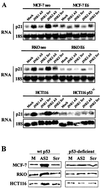
FIG. 8
JNK2AS treatment results in induction of p21_Cip1/Waf1_ expression in cells with normal p53 function (neo-transfected MCF-7 and RKO, and HCT166 cells) but not in p53-deficient cells (E6-expressing MCF-7 and RKO, and HCT116 p53−/− cells). (A) Total RNA was extracted from cells 24 h following treatment with 0.3 μM antisense (AS) or control (Scr) oligonucleotides. RNA samples were analyzed by Northern analysis using an oligonucleotide probe specific for human p21_Cip1/Waf1_. Following analysis of p21_Cip1/Waf1_ expression, Northern blots were stripped and reprobed with an oligonucleotide complementary to 18S rRNA to verify equal loading and transfer of RNA samples. (B) p21_Cip1/Waf1_ expression was examined by Western blot analyses 24 h following treatment with 0.3 μM JNK2AS or JNKScr oligonucleotides (as described in the legend to Fig. 3).
FIG. 9
HCT116 human colorectal carcinoma cells lacking p21_Cip1/Waf1_ expression display enhanced sensitivity to JNK2AS treatment. Parental HCT116 cells with normal p21_Cip1/Waf1_ expression (p21 +/+) and derivative lines in which one (p21 +/−) or both (p21 −/−) p21_Cip1/Waf1_ alleles have been disrupted through homologous recombination were examined by FACS analysis for apoptotic cells 24 h following treatment with JNK1AS or JNK2AS oligonucleotides.
Similar articles
- c-Jun N-terminal kinase is essential for growth of human T98G glioblastoma cells.
Potapova O, Gorospe M, Bost F, Dean NM, Gaarde WA, Mercola D, Holbrook NJ. Potapova O, et al. J Biol Chem. 2000 Aug 11;275(32):24767-75. doi: 10.1074/jbc.M904591199. J Biol Chem. 2000. PMID: 10825181 - Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21(WAF1/Cip1) and mediate growth arrest.
Zuo Z, Dean NM, Honkanen RE. Zuo Z, et al. J Biol Chem. 1998 May 15;273(20):12250-8. doi: 10.1074/jbc.273.20.12250. J Biol Chem. 1998. PMID: 9575175 - The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function.
Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. Gizatullin F, et al. Cancer Res. 2006 Aug 1;66(15):7668-77. doi: 10.1158/0008-5472.CAN-05-3353. Cancer Res. 2006. PMID: 16885368 - Nocodazole-induced p53-dependent c-Jun N-terminal kinase activation reduces apoptosis in human colon carcinoma HCT116 cells.
Zhang H, Shi X, Zhang QJ, Hampong M, Paddon H, Wahyuningsih D, Pelech S. Zhang H, et al. J Biol Chem. 2002 Nov 15;277(46):43648-58. doi: 10.1074/jbc.M203214200. Epub 2002 Sep 6. J Biol Chem. 2002. PMID: 12221076 - The Jun kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung carcinoma cells.
Bost F, McKay R, Bost M, Potapova O, Dean NM, Mercola D. Bost F, et al. Mol Cell Biol. 1999 Mar;19(3):1938-49. doi: 10.1128/MCB.19.3.1938. Mol Cell Biol. 1999. PMID: 10022881 Free PMC article.
Cited by
- Molecular mechanisms underlying the time-dependent autophagy and apoptosis induced by nutrient depletion in multiple myeloma: a pilot study.
Liu Y, Chen Y, Wen L, Cui G. Liu Y, et al. J Huazhong Univ Sci Technolog Med Sci. 2012 Feb;32(1):1-8. doi: 10.1007/s11596-012-0001-2. Epub 2012 Jan 27. J Huazhong Univ Sci Technolog Med Sci. 2012. PMID: 22282237 - Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells.
Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA, Hollenberg MD, Marshall J, McCulloch CA, Abreu MT, Chow CW, Downey GP. Suzuki T, et al. Am J Respir Cell Mol Biol. 2005 Sep;33(3):231-47. doi: 10.1165/rcmb.2005-0109OC. Epub 2005 May 12. Am J Respir Cell Mol Biol. 2005. PMID: 15891109 Free PMC article. - Anticancer activity of marine sponge Hyrtios sp. extract in human colorectal carcinoma RKO cells with different p53 status.
Lim HK, Bae W, Lee HS, Jung J. Lim HK, et al. Biomed Res Int. 2014;2014:413575. doi: 10.1155/2014/413575. Epub 2014 Aug 27. Biomed Res Int. 2014. PMID: 25243139 Free PMC article. - Beryllium sulfate induces p21 CDKN1A expression and a senescence-like cell cycle arrest in susceptible cancer cell types.
Gorjala P, Gary RK. Gorjala P, et al. Biometals. 2010 Dec;23(6):1061-73. doi: 10.1007/s10534-010-9352-y. Epub 2010 Jun 12. Biometals. 2010. PMID: 20549306 Free PMC article. - Methylsulfonylmethane Induces p53 Independent Apoptosis in HCT-116 Colon Cancer Cells.
Karabay AZ, Koc A, Ozkan T, Hekmatshoar Y, Sunguroglu A, Aktan F, Buyukbingol Z. Karabay AZ, et al. Int J Mol Sci. 2016 Jul 15;17(7):1123. doi: 10.3390/ijms17071123. Int J Mol Sci. 2016. PMID: 27428957 Free PMC article.
References
- Angel P, Karin M. The role of Jun, Fos, and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
- Antonyak M A, Moscatello D K, Wong A J. Constitutive activation of c-Jun N-terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem. 1998;273:2817–2822. - PubMed
- Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. - PubMed
- Bost F, Dean N, McKay R, Mercola D. Activation of the Jun kinase/stress-activated protein kinase pathway is required for EGF-autocrine stimulated growth of human A549 lung carcinoma cells. J Biol Chem. 1997;272:33422–33429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous