Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice - PubMed (original) (raw)
Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice
F N Ziyadeh et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Emerging evidence suggests that transforming growth factor-beta (TGF-beta) is an important mediator of diabetic nephropathy. We showed previously that short-term treatment with a neutralizing monoclonal anti-TGF-beta antibody (alphaT) in streptozotocin-diabetic mice prevents early changes of renal hypertrophy and increased matrix mRNA. To establish that overactivity of the renal TGF-beta system mediates the functional and structural changes of the more advanced stages of nephropathy, we tested whether chronic administration of alphaT prevents renal insufficiency and glomerulosclerosis in the db/db mouse, a model of type 2 diabetes that develops overt nephropathy. Diabetic db/db mice and nondiabetic db/m littermates were treated intraperitoneally with alphaT or control IgG, 300 microgram three times per week for 8 wk. Treatment with alphaT, but not with IgG, significantly decreased the plasma TGF-beta1 concentration without decreasing the plasma glucose concentration. The IgG-treated db/db mice developed albuminuria, renal insufficiency, and glomerular mesangial matrix expansion associated with increased renal mRNAs encoding alpha1(IV) collagen and fibronectin. On the other hand, treatment with alphaT completely prevented the increase in plasma creatinine concentration, the decrease in urinary creatinine clearance, and the expansion of mesangial matrix in db/db mice. The increase in renal matrix mRNAs was substantially attenuated, but the excretion of urinary albumin factored for creatinine clearance was not significantly affected by alphaT treatment. We conclude that chronic inhibition of the biologic actions of TGF-beta with a neutralizing monoclonal antibody in db/db mice prevents the glomerulosclerosis and renal insufficiency resulting from type 2 diabetes.
Figures
Figure 1
Increased glomerular TGF-β1 mRNA in diabetic db/db mice. In situ hybridization of kidney sections: (A) antisense TGF-β1 riboprobe in 16-wk-old control db/m mouse showing minimal hybridization signal; (B) antisense TGF-β1 riboprobe in 16-wk-old diabetic db/db mouse showing increased hybridization signal in the mesangial area; (C) sense TGF-β1 riboprobe used as negative control in diabetic db/db mouse showing absence of hybridization signal; and (D) quantitative analysis of area of TGF-β1 hybridization signal in db/db vs. db/m mice. Twenty glomeruli were studied in each group, and the values presented are the mean ± SE for four mice in each group. *, P < 0.05 vs. db/m.
Figure 2
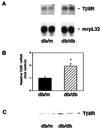
Up-regulation of renal TGF-β type II receptor in db/db mice. (A) Representative Northern blot showing increased TGF-β type II receptor mRNA in db/db mouse kidney compared with db/m mouse kidney. (B) Summary of densitometric analyses of receptor/mrpL32 mRNA ratios (mean ± SE, n = 5 for each group). The relative ratio in the db/m group is assigned a value of 1. *, P < 0.05 vs. db/m. (C) Representative immunoblot showing increased TGF-β type II receptor protein in db/db mouse kidney compared with db/m mouse kidney.
Figure 3
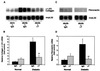
Kidney matrix gene expression in diabetic mice treated with anti-TGF-β antibody. A representative Northern blot of kidney RNA probed with α1(IV) collagen cDNA (A) and fibronectin cDNA (C) and then with ribosomal mrpL32. Each lane represents RNA from individual mice (db/m or db/db), treated with either IgG or anti-TGF-β antibody (αT). Summary of densitometric analyses of α1(IV) collagen/mrpL32 mRNA ratios (B) and fibronectin/mrpL32 mRNA ratios (D) in the four treatment groups (mean ± SE, n = 8 for each IgG group and n = 9 for each αT group). The relative mRNA ratio in the normal-IgG group is assigned a value of 1. *, P < 0.05 vs. normal-IgG, **, P < 0.05 vs. diabetic-IgG.
Figure 4
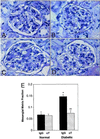
Anti-TGF-β antibody therapy significantly prevents mesangial matrix expansion in diabetic mice. Representative photomicrographs of PAS-stained kidney sections from: (A) normal db/m mouse treated with control IgG; (B) normal db/m mouse treated with anti-TGF-β antibody (αT); (C) diabetic db/db mouse treated with control IgG; and (D) diabetic db/db mouse treated with αT. Note the diffusely expanded extracellular mesangial matrix in the diabetic mouse treated with IgG and the marked prevention of this expansion in the diabetic mouse treated with αT. Quantitative measurement of extracellular mesangial matrix expansion (E) is expressed as PAS-positive mesangial material per total glomerular tuft cross-sectional area. An average value was obtained from analyses of 30 glomeruli per mouse. Data are mean ± SE, n = 8 for each IgG group and n = 9 for each αT group. *, P < 0.05 vs. normal-IgG, **, P < 0.05 vs. diabetic-IgG.
Figure 5
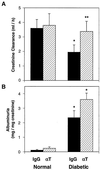
Anti-TGF-β antibody therapy normalizes renal function but not albuminuria in diabetic mice. (A) Creatinine clearance in normal db/m and diabetic db/db groups treated with either IgG or anti-TGF-β antibody (αT). Clearance was calculated based on urine volumes and plasma and urine creatinine concentrations. ELISA specific for mouse albumin was used to assess albuminuria in 24-h urine collections, which was standardized per mg creatinine. (B) The increased excretion of albumin in db/db mice treated with IgG (vs. db/m mice) remained persistently elevated in db/db mice treated with αT. Data are mean ± SE, n = 8 for each IgG group and n = 9 for each αT group. *, P < 0.05 vs. normal-IgG or normal-αT, **, P < 0.05 vs. diabetic-IgG.
Comment in
- Transforming growth factor beta contributes to progressive diabetic nephropathy.
Reeves WB, Andreoli TE. Reeves WB, et al. Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):7667-9. doi: 10.1073/pnas.97.14.7667. Proc Natl Acad Sci U S A. 2000. PMID: 10884396 Free PMC article. No abstract available.
Similar articles
- Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF-beta type II receptor expression.
Han DC, Isono M, Chen S, Casaretto A, Hong SW, Wolf G, Ziyadeh FN. Han DC, et al. Kidney Int. 2001 Apr;59(4):1315-23. doi: 10.1046/j.1523-1755.2001.0590041315.x. Kidney Int. 2001. PMID: 11260392 - Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse.
Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Hong SW, et al. Am J Pathol. 2001 May;158(5):1653-63. doi: 10.1016/s0002-9440(10)64121-1. Am J Pathol. 2001. PMID: 11337363 Free PMC article. - The key role of the transforming growth factor-beta system in the pathogenesis of diabetic nephropathy.
Chen S, Hong SW, Iglesias-de la Cruz MC, Isono M, Casaretto A, Ziyadeh FN. Chen S, et al. Ren Fail. 2001 May-Jul;23(3-4):471-81. doi: 10.1081/jdi-100104730. Ren Fail. 2001. PMID: 11499562 Review. - Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up.
Chen S, Jim B, Ziyadeh FN. Chen S, et al. Semin Nephrol. 2003 Nov;23(6):532-43. doi: 10.1053/s0270-9295(03)00132-3. Semin Nephrol. 2003. PMID: 14631561 Review.
Cited by
- MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy.
Koga K, Yokoi H, Mori K, Kasahara M, Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, Toda N, Saleem MA, Sugawara A, Nakao K, Yanagita M, Mukoyama M. Koga K, et al. Diabetologia. 2015 Sep;58(9):2169-80. doi: 10.1007/s00125-015-3642-4. Epub 2015 Jun 11. Diabetologia. 2015. PMID: 26063197 - Anti-TGF-β Antibody, 1D11, Ameliorates Glomerular Fibrosis in Mouse Models after the Onset of Proteinuria.
Liang X, Schnaper HW, Matsusaka T, Pastan I, Ledbetter S, Hayashida T. Liang X, et al. PLoS One. 2016 May 17;11(5):e0155534. doi: 10.1371/journal.pone.0155534. eCollection 2016. PLoS One. 2016. PMID: 27187580 Free PMC article. - ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells.
Zhang M, Fraser D, Phillips A. Zhang M, et al. Am J Pathol. 2006 Oct;169(4):1282-93. doi: 10.2353/ajpath.2006.050921. Am J Pathol. 2006. PMID: 17003485 Free PMC article. - RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy.
Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM. Wendt TM, et al. Am J Pathol. 2003 Apr;162(4):1123-37. doi: 10.1016/S0002-9440(10)63909-0. Am J Pathol. 2003. PMID: 12651605 Free PMC article. - Aberrant DNA methylation of Tgfb1 in diabetic kidney mesangial cells.
Oba S, Ayuzawa N, Nishimoto M, Kawarazaki W, Ueda K, Hirohama D, Kawakami-Mori F, Shimosawa T, Marumo T, Fujita T. Oba S, et al. Sci Rep. 2018 Nov 5;8(1):16338. doi: 10.1038/s41598-018-34612-3. Sci Rep. 2018. PMID: 30397232 Free PMC article.
References
- Ziyadeh F N. Am J Kidney Dis. 1993;22:736–744. - PubMed
- Ziyadeh F N. Kidney Int Suppl. 1996;54:S10–S13. - PubMed
- Ziyadeh F N. Miner Electrolyte Metab. 1995;21:292–302. - PubMed
- Ayo S H, Radnik R A, Glass W F, II, Garoni J A, Rampt E R, Appling D R, Kreisberg J I. Am J Physiol. 1991;260:F185–F191. - PubMed
- Danne T, Spiro M J, Spiro R G. Diabetes. 1993;42:170–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-45193/DK/NIDDK NIH HHS/United States
- R01 DK044513/DK/NIDDK NIH HHS/United States
- DK-44513/DK/NIDDK NIH HHS/United States
- DK-54608/DK/NIDDK NIH HHS/United States
- T32 DK007006/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous