Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation - PubMed (original) (raw)
Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation
A W Goldrath et al. J Exp Med. 2000.
Abstract
In a depleted lymphoid compartment, naive T cells begin a slow proliferation that is independent of cognate antigen yet requires recognition of major histocompatibility complex-bound self-peptides. We have followed the phenotypic and functional changes that occur when naive CD8(+) T cells undergo this type of expansion in a lymphopenic environment. Naive T cells undergoing homeostasis-driven proliferation convert to a phenotypic and functional state similar to that of memory T cells, yet distinct from antigen-activated effector T cells. Naive T cells dividing in a lymphopenic host upregulate CD44, CD122 (interleukin 2 receptor beta) and Ly6C expression, acquire the ability to rapidly secrete interferon gamma, and become cytotoxic effectors when stimulated with cognate antigen. The conversion of naive T cells to cells masquerading as memory cells in response to a homeostatic signal does not represent an irreversible differentiation. Once the cellularity of the lymphoid compartment is restored and the T cells cease their division, they regain the functional and phenotypic characteristics of naive T cells. Thus, homeostasis-driven proliferation provides a thymus-independent mechanism for restoration of the naive compartment after a loss of T cells.
Figures
Figure 1
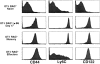
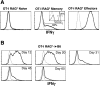
Naive T cells undergoing homeostasis-driven proliferation acquire a memory phenotype. Expression of CD44, Ly6C, and CD122 on OT-I RAG−/− T cells transferred 17 d earlier into an irradiated B6 host compared with naive, memory, and effector OT-I RAG−/− CD8+ T cells. Histogram plots show expression of the indicated marker for Vα2+Vβ5+CD8+ gated cells. Before transfer, OT-I CD8+ cells were depleted of CD44hi and Ly6Chi cells. Memory cells were recovered from irradiated B6 hosts that had received 3 × 106 OT-I RAG−/− cells, followed by infection with vaccinia virus encoding OVA >90 d earlier. OT-I effectors were analyzed on day 5 after in vitro stimulation with OVAp-coated, irradiated splenocyte feeder cells.
Figure 2
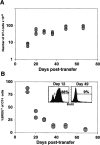
Kinetics of homeostasis-driven proliferation. Irradiated B6 hosts received 3 × 106 OT-I RAG−/− T cells. (A) Total numbers of OT-I T cells were estimated by determining the number of Vα2+Vβ5+CD8+ T cells recovered from the pooled spleen and lymph nodes (four mice per time point). At day 28 of transfer, 25%+/−3.4 of recovered cells are OT-I, 6.8%+/−0.5 are host-derived CD8+ T cells, and 21%+/−1.1 are host-derived CD4+ T cells. (B) The level of proliferation by transferred OT-I cells was determined at each time point by BrdU incorporation. Mice were given BrdU for 7 d before each time point (two mice per time point). The percentage of Vα2+CD8+ cells that had incorporated BrdU is shown. The insets show BrdU staining for Vα2+CD8+ gated cells 12 and 49 d after transfer.
Figure 3
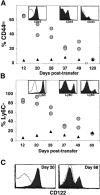
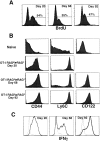
OT-I T cells revert to a naive phenotype after homeostasis-driven proliferation. Expression levels of CD44, Ly6C, and CD122 for Vα2+Vβ5+CD8+ gated naive OT-I T cells analyzed on the same day (▴) or OT-I RAG−/− cells recovered from two different irradiated B6 hosts (circles) at the indicated time points are graphed. (A) Percentage of CD44hi of Vα2+Vβ5+CD8+ gated T cells. Histogram insets show CD44 expression for transferred OT-I cells at days 20, 49, and 120 (filled histogram) overlayed with naive OT-I cells (open histogram). (B) Percentage of Ly6C+ of Vα2+Vβ5+CD8+ gated T cells. Histogram insets show Ly6C expression for transferred OT-I cells at days 20, 45, and 120 (filled histogram) overlayed with naive OT-I T cells (open histogram). (C) Expression of CD122 on naive OT-I cells (open histogram) and OT-I cells 20 and 68 d after transfer (filled histogram).
Figure 4
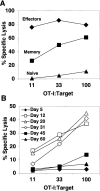
OT-I cells undergoing homeostasis-driven proliferation transiently acquire ex vivo CTL activity. Percentage of specific lysis of OVAp-pulsed syngeneic targets was determined in a 6-h CTL assay. Lysis of target cells in the absence of OVAp was <6%. (A) Cytotoxic activity is shown for in vitro–stimulated OT-I effectors, in vivo–derived memory cells, and naive OT-I cells. (B) Cytotoxic activity is shown for OT-I cells recovered from spleen and lymph nodes of irradiated B6 hosts at the indicated time points after transfer. The E/T ratios were normalized for the number of OT-I (Vα2+Vβ5+CD8+) cells in all samples. Fresh, naive OT-I RAG−/− control cells and OT-I effector cells were run at every time point. Naive cells never gave lysis >10% at the highest E/T, and effector cells always gave lysis >60% at 11:1. CTL activity of memory OT-I cells was assayed on four occasions and was always intermediate between naive and effector activity.
Figure 5
OT-I cells undergoing homeostasis-driven proliferation rapidly make IFN-γ at early time points. Pooled spleen and lymph node cells were incubated with (bold lines) or without (dotted lines) OVAp for 8 h, and were stained with anti–IFN-γ. Histogram overlays are shown for CD8+ gated cells, as the cells cannot be stained for either Vα2 or Vβ5 expression because the antigen pulse induces TCR downregulation. (A) IFN-γ levels for naive, memory, and effector OT-I cells. The inset shows tetramer staining, Kb-OVAp (bold line), or a control Kb-peptide (dotted line), of CD8+ gated memory cells before peptide stimulation. (B) IFN-γ staining for CD8+ gated cells recovered from irradiated B6 hosts at the indicated time points.
Figure 6
Proliferation of OT-I cells in RAG−/− recipients. (A) BrdU incorporation is shown for Vα2+CD8+ gated cells recovered from pooled spleen and lymph nodes of RAG−/− hosts given BrdU for 7 d before the indicated time point. (B) Phenotype of Vα2+Vβ5+CD8+ gated T cells from pooled spleen and lymph nodes of naive OT-I RAG−/− mice or OT-I RAG−/− cells recovered from RAG−/− hosts at the indicated time points. (C) IFN-γ production by CD8+ cells recovered from RAG−/− hosts at the indicated time points after 8 h of stimulation with OVAp (bold lines) or media alone (dotted lines).
Comment in
- Homeostatic T cell proliferation: how far can T cells be activated to self-ligands?
Surh CD, Sprent J. Surh CD, et al. J Exp Med. 2000 Aug 21;192(4):F9-F14. doi: 10.1084/jem.192.4.f9. J Exp Med. 2000. PMID: 10952731 Free PMC article. Review. No abstract available.
Similar articles
- Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells.
Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Cho BK, et al. J Exp Med. 2000 Aug 21;192(4):549-56. doi: 10.1084/jem.192.4.549. J Exp Med. 2000. PMID: 10952724 Free PMC article. - Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments.
Ge Q, Hu H, Eisen HN, Chen J. Ge Q, et al. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2989-94. doi: 10.1073/pnas.052714099. Proc Natl Acad Sci U S A. 2002. PMID: 11880642 Free PMC article. - Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences.
Zimmermann C, Prévost-Blondel A, Blaser C, Pircher H. Zimmermann C, et al. Eur J Immunol. 1999 Jan;29(1):284-90. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. Eur J Immunol. 1999. PMID: 9933110 - Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes.
Ramanathan S, Gagnon J, Dubois S, Forand-Boulerice M, Richter MV, Ilangumaran S. Ramanathan S, et al. Crit Rev Immunol. 2009;29(3):219-39. doi: 10.1615/critrevimmunol.v29.i3.30. Crit Rev Immunol. 2009. PMID: 19538136 Review. - Stem cell-like plasticity of naïve and distinct memory CD8+ T cell subsets.
Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stemberger C, et al. Semin Immunol. 2009 Apr;21(2):62-8. doi: 10.1016/j.smim.2009.02.004. Epub 2009 Mar 9. Semin Immunol. 2009. PMID: 19269852 Review.
Cited by
- CD8+ T-Cell Memory: The Why, the When, and the How.
Turner SJ, Bennett TJ, La Gruta NL. Turner SJ, et al. Cold Spring Harb Perspect Biol. 2021 May 3;13(5):a038661. doi: 10.1101/cshperspect.a038661. Cold Spring Harb Perspect Biol. 2021. PMID: 33648987 Free PMC article. Review. - Novel multiparameter correlates of Coxiella burnetii infection and vaccination identified by longitudinal deep immune profiling.
Reeves PM, Raju Paul S, Baeten L, Korek SE, Yi Y, Hess J, Sobell D, Scholzen A, Garritsen A, De Groot AS, Moise L, Brauns T, Bowen R, Sluder AE, Poznansky MC. Reeves PM, et al. Sci Rep. 2020 Aug 7;10(1):13311. doi: 10.1038/s41598-020-69327-x. Sci Rep. 2020. PMID: 32770104 Free PMC article. - IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens.
Deshpande P, Cavanagh MM, Le Saux S, Singh K, Weyand CM, Goronzy JJ. Deshpande P, et al. J Immunol. 2013 Feb 15;190(4):1416-23. doi: 10.4049/jimmunol.1201620. Epub 2013 Jan 16. J Immunol. 2013. PMID: 23325887 Free PMC article. - Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions.
Chakraverty R, Eom HS, Sachs J, Buchli J, Cotter P, Hsu R, Zhao G, Sykes M. Chakraverty R, et al. Blood. 2006 Sep 15;108(6):2106-13. doi: 10.1182/blood-2006-03-007427. Epub 2006 Jun 6. Blood. 2006. PMID: 16757687 Free PMC article. - Inducible IL-7 Hyperexpression Influences Lymphocyte Homeostasis and Function and Increases Allograft Rejection.
Schreiber M, Weigelt M, Karasinsky A, Anastassiadis K, Schallenberg S, Petzold C, Bonifacio E, Kretschmer K, Hommel A. Schreiber M, et al. Front Immunol. 2019 Apr 10;10:742. doi: 10.3389/fimmu.2019.00742. eCollection 2019. Front Immunol. 2019. PMID: 31024566 Free PMC article.
References
- Tanchot C., Rosado M.M., Agenes F., Freitas A.A., Rocha B. Lymphocyte homeostasis. Semin. Immunol. 1997;9:331–337. - PubMed
- Goldrath A.W., Bevan M.J. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. - PubMed
- Tanchot C., Lemonnier F.A., Perarnau B., Freitas A.A., Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. - PubMed
- Nesic D., Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J. Immunol. 1998;160:3705–3712. - PubMed
- Takeda S., Rodewald H.-R., Arakawa H., Bluethmann H., Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous