The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex - PubMed (original) (raw)
The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex
R A Heil-Chapdelaine et al. J Cell Biol. 2000.
Abstract
In budding yeast, the mitotic spindle moves into the neck between the mother and bud via dynein-dependent sliding of cytoplasmic microtubules along the cortex of the bud. How dynein and microtubules interact with the cortex is unknown. We found that cells lacking Num1p failed to exhibit dynein-dependent microtubule sliding in the bud, resulting in defective mitotic spindle movement and nuclear segregation. Num1p localized to the bud cortex, and that localization was independent of microtubules, dynein, or dynactin. These data are consistent with Num1p being an essential element of the cortical attachment mechanism for dynein-dependent sliding of microtubules in the bud.
Figures
Figure 1
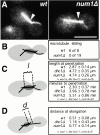
Microtubule and spindle behavior in _num1_Δ mutants. A, Frames from videos of GFP-labeled microtubules during movement of the spindle into the bud neck (see http://www.jcb.org/cgi/content/full/151/6/1337/DC1). The arrow marks the bud-neck. The cytoplasmic microtubule is “plastered” along the bud cortex in the wt cell, but not in the _num1_Δ cell. Bar, 5 μm. The accompanying videos show sliding in a wt cell and lack of sliding in a _num1_Δ cell. B, Frequency of cytoplasmic microtubules sliding along the cortex in wt and _num1_Δ cells. C, The length of the spindle 1 min before it penetrated the neck. Spindles in _num1_Δ cells were longer than spindles in wt cells, similar to spindles in dhc1_Δ cells (num1_Δ vs. wt, P = 2.6 × 10−7; _num1_Δ vs. _dhc1_Δ, P = 0.97). Also, the time from initiation of spindle elongation to penetration of the neck was determined. Spindles took longer to enter the neck in _num1_Δ cells than in wt cells (P = 2 × 10−3), but no longer than in _dhc1_Δ cells (P = 0.19). D, The distance of the spindle from the neck at initiation of spindle elongation. None of the differences are statistically significant (_num1_Δ vs. wt, P = 0.13; _dhc1_Δ vs. wt, P = 0.17; _num1_Δ vs. _dhc1_Δ, P = 0.41).
Figure 2
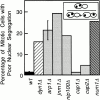
Nuclear segregation in _num1_Δ, dynein, and dynactin mutants. The frequency of cells with both nuclei in the mother as a percentage of total mitotic events is plotted. Error bars represent SEM (wt, n = 7; _arp1_Δ, n = 5).
Figure 3
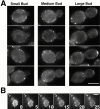
Num1p localization. A, Representative cells expressing Num1–GFP are shown. Bar, 5 μm. B, Num1–GFP localization in a single cell over time. 16 focal planes were collected every minute in diploid cells expressing Num1–GFP and projected to two dimensions. Shown are frames at 5 min intervals. At t = 0 the mother has bright cortical spots and the bud has no staining. The spots in the mother do not move. At 15 min, a small amount of fluorescence staining appears at the bud tip. The bud tip staining enlarges over time (arrow). Bar, 5 μm.
Figure 4
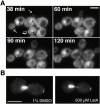
Localization of Num1p without microtubules and without filamentous actin. A, Localization of Num1–GFP does not require microtubules. Log phase diploid cells expressing GFP–tubulin and Num1–GFP were treated with 25 μM nocodazole at t = 0 min to depolymerize microtubules, and images were collected at 30-min intervals. The black arrows indicate residual GFP–tubulin fluorescence at spindle pole bodies. The white arrows indicate growing buds. The larger of the two growing buds has dim cortical fluorescence that increases over time. The smaller of the two growing buds begins to show cortical fluorescence at 90 min after nocodazole addition, and a bright cortical crescent appears at 120 min. The open arrow shows a crescent of spots at the tip of a large budded cell (mother cell is above and to the right of the bud). Cell separation occurs between 38 and 60 min, manifested by a shift in the position of the bud relative to the mother. Fluorescence remains at what was the tip of the bud throughout the time course. Bar, 5 μm. These results are identical to those for cells not treated with nocodazole (data not shown). B, Maintenance of Num1–GFP to the bud tip does not require filamentous actin. Cells expressing Num1–GFP and GFP–tubulin were treated with 200 mM HU to arrest them as large budded cells with short mitotic spindles. The cells were treated with 1% DMSO as a control or 500 μM latrunculin A to depolymerize actin. Both DMSO-treated and latrunculin A-treated cells show bright fluorescence at the tip of the bud after 10 min of treatment. Similar results were obtained from 5 to 30 min after treatment. Bar, 5 μm.
Similar articles
- The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast.
Lee WL, Oberle JR, Cooper JA. Lee WL, et al. J Cell Biol. 2003 Feb 3;160(3):355-64. doi: 10.1083/jcb.200209022. J Cell Biol. 2003. PMID: 12566428 Free PMC article. - Dynamic positioning of mitotic spindles in yeast: role of microtubule motors and cortical determinants.
Yeh E, Yang C, Chin E, Maddox P, Salmon ED, Lew DJ, Bloom K. Yeh E, et al. Mol Biol Cell. 2000 Nov;11(11):3949-61. doi: 10.1091/mbc.11.11.3949. Mol Biol Cell. 2000. PMID: 11071919 Free PMC article. - Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae.
Adames NR, Cooper JA. Adames NR, et al. J Cell Biol. 2000 May 15;149(4):863-74. doi: 10.1083/jcb.149.4.863. J Cell Biol. 2000. PMID: 10811827 Free PMC article. - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian. - Mitotic motors in Saccharomyces cerevisiae.
Hildebrandt ER, Hoyt MA. Hildebrandt ER, et al. Biochim Biophys Acta. 2000 Mar 17;1496(1):99-116. doi: 10.1016/s0167-4889(00)00012-4. Biochim Biophys Acta. 2000. PMID: 10722880 Review.
Cited by
- Kar9p-independent microtubule capture at Bud6p cortical sites primes spindle polarity before bud emergence in Saccharomyces cerevisiae.
Segal M, Bloom K, Reed SI. Segal M, et al. Mol Biol Cell. 2002 Dec;13(12):4141-55. doi: 10.1091/mbc.02-05-0067. Mol Biol Cell. 2002. PMID: 12475941 Free PMC article. - Cortical dynein pulling mechanism is regulated by differentially targeted attachment molecule Num1.
Omer S, Greenberg SR, Lee WL. Omer S, et al. Elife. 2018 Aug 7;7:e36745. doi: 10.7554/eLife.36745. Elife. 2018. PMID: 30084355 Free PMC article. - Temporal control of contact site formation reveals a relationship between mitochondrial division and Num1-mediated mitochondrial tethering.
Harper CS, Casler JC, Lackner LL. Harper CS, et al. Mol Biol Cell. 2023 Oct 1;34(11):ar108. doi: 10.1091/mbc.E23-05-0168. Epub 2023 Aug 16. Mol Biol Cell. 2023. PMID: 37585290 Free PMC article. - Spatial signals link exit from mitosis to spindle position.
Falk JE, Tsuchiya D, Verdaasdonk J, Lacefield S, Bloom K, Amon A. Falk JE, et al. Elife. 2016 May 11;5:e14036. doi: 10.7554/eLife.14036. Elife. 2016. PMID: 27166637 Free PMC article. - Loss of Num1-mediated cortical dynein anchoring negatively impacts respiratory growth.
White AJ, Harper CS, Rosario EM, Dietz JV, Addis HG, Fox JL, Khalimonchuk O, Lackner LL. White AJ, et al. J Cell Sci. 2022 Nov 1;135(21):jcs259980. doi: 10.1242/jcs.259980. Epub 2022 Oct 31. J Cell Sci. 2022. PMID: 36185004 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases