The transcription factor Sox10 is a key regulator of peripheral glial development - PubMed (original) (raw)
The transcription factor Sox10 is a key regulator of peripheral glial development
S Britsch et al. Genes Dev. 2001.
Abstract
The molecular mechanisms that determine glial cell fate in the vertebrate nervous system have not been elucidated. Peripheral glial cells differentiate from pluripotent neural crest cells. We show here that the transcription factor Sox10 is a key regulator in differentiation of peripheral glial cells. In mice that carry a spontaneous or a targeted mutation of Sox10, neuronal cells form in dorsal root ganglia, but Schwann cells or satellite cells are not generated. At later developmental stages, this lack of peripheral glial cells results in a severe degeneration of sensory and motor neurons. Moreover, we show that Sox10 controls expression of ErbB3 in neural crest cells. ErbB3 encodes a Neuregulin receptor, and down-regulation of ErbB3 accounts for many changes in development of neural crest cells observed in Sox10 mutant mice. Sox10 also has functions not mediated by ErbB3, for instance in the melanocyte lineage. Phenotypes observed in heterozygous mice that carry a targeted Sox10 null allele reproduce those observed in heterozygous Sox10(Dom) mice. Haploinsufficiency of Sox10 can thus cause pigmentation and megacolon defects, which are also observed in Sox10(Dom)/+ mice and in patients with Waardenburg-Hirschsprung disease caused by heterozygous SOX10 mutations.
Figures
Figure 1
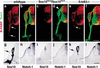
Targeted deletion of Sox10 and expression of the Sox10lacZ allele in mice. (a) Schematic representation of the targeting strategy employed to disrupt the Sox10 gene. Shown are the targeting vector (top), the wild-type Sox10 locus (middle) and the mutated locus after homologous recombination (bottom). Noncoding and coding exons are represented by black or open boxes, respectively. Restriction sites for _Spe_I (P) and _Nco_I (N) as well as the location of the 5′ probe are indicated. (b) Southern blot analysis of DNA from adult heterozygous (+/−) and wild-type (+/+) mice after digestion with _Nco_I/_Spe_I. The expected size of the fragments detected by the probe used for hybridization are shown (a). (c) RT–PCR analysis of wild-type (+/+), heterozygous (+/−) and homozygous (−/−) embryos at E12.5 using primers specific for the coding region of Sox10. Results from 20, 23, and 26 cycles are shown for each genotype. Sox10 lacZ/+ (d–f) and Sox10lacZ/Sox10lacZ (g–i) embryos at E8.5 (d,g), E10.5 (e,h), and E12.5 (f,i) after X-gal staining.
Figure 2
Sox10 controls expression of ErbB3. Whole-mount in situ hybridizations of wild-type (a–d), Sox10Dom/Sox10Dom (e–l), and ErbB3_–/−_ (m–p) embryos with probes specific for Sox10 (top), and ErbB3 (bottom). Shown are lateral (a,b,e,f,m,n) views of E10.5 embryos and the corresponding vibratome sections (c,d,g,h,o,p) on forelimb levels. Lateral (i,j) and dorsal (k,l) views of Sox10Dom/Sox10Dom embryos at E9.0. Arrows in f, j, and l point toward _ErbB3_-expressing cells close to the neural tube in Sox10Dom/Sox10Dom embryos; arrows in c, g, and o point toward the anlage of the primary sympathetic ganglion chain (sy); arrowheads in d, f, h, and j point toward the myotome that also expresses ErbB3. Bars (a,b,e,f,m,n) 1 mm; (c,d,g,h,o,p) 100 μm; (i–l) 300 μm.
Figure 3
Sox10 increases endogenous ErbB3 expression in N2A cells. (a,b) RT–PCR analysis of mRNA obtained from N2A cells that express Sox10, Sox11, or a mutant version of Sox10 (FL, wild-type cDNAs; Sox10fs, Sox10Dom mutant; Kuhlbrodt et al. 1998b) in an inducible manner. Doxycycline (DOXA) was used to induce the expression of the transcription factors (+), which was compared to the uninduced state (−). ErbB3 transcript levels were compared in the various transfectants by semiquantitative PCR, using increasing numbers of amplification cycles (20, 23, and 26); in addition, the expression of GAPDH, Sox10, and Sox11 was monitored. (c) Northern blot analysis on 2 μg of poly(A)+ RNA from uninduced (left) and doxycycline-treated (right) N2A cells that express Sox10 in an inducible manner. The ErbB3-specific signal is indicated by an asterisk.
Figure 4
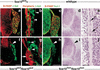
Sox10 controls peripheral glial cell development. (a–f) Immunohistological analysis of wild-type (a,b), Sox10Dom/Sox10Dom (c,d), and _ErbB3_−/−(e,f) embryos at E11.5 on lumbar axial levels, using antibodies against B-FABP (red) and TuJ-1(green) to visualize peripheral glial cells and neuronal cells, respectively. B-FABP antibody signals (a,c,e) and an overlay of B-FABP and TuJ-1 antibody signals (b,d,f) are shown. The arrowhead in d points towards the abnormally shaped dorsal root entry zones in Sox10Dom/Sox10Dom mutants; the arrows point towards B-FABP-positive Schwann cell precursors that line spinal nerves in control and _ErbB3_−/− mice. (g–l) In situ hybridization of wild-type (g,h), Sox10Dom/Sox10Dom (i,j), and _ErbB3_−/− (k,l) embryos at E11.5 with probes specific for Sox10 (g,i,k) or Notch-1 (h,j,l). Sections on lumbar axial levels are shown. Bars (a–f) 100 μm; (g–l) 150 μm.
Figure 5
Appearance of dorsal root ganglia in homozygous Sox10 mutant animals. Histological and immunohistological analyses of dorsal root ganglia and spinal nerves in Sox10 lacZ/+ (a,b), wild-type (c–e), Sox10lacZ/Sox10lacZ (f,g), and Sox10Dom/Sox10Dom (h–j) animals at E11.5 (a,b,f,g) and E12.5 (c–e,h–j). (a,f) Immunohistological analysis using antibodies against B-FABP (red) and β-galactosidase (green). Note that B-FABP and β-galactosidase from the Sox10–lacZ reporter are coexpressed (yellow) in satellite glia of dorsal root ganglia of control mice. B-FABP is not expressed in Sox10lacZ/Sox10lacZ mice, although β-galactosidase-positive cells persist. (b,g). High-magnification of ventral roots stained with antibodies against peripherin (red) and β-galactosidase (green). Arrowheads point towards cells that express β-galactosidase; arrows point towards neuronal cells expressing peripherin. Note that the two proteins are coexpressed in neither control nor homozygous mutant mice, indicating that the β-galactosidase-positive precursor cells have not adopted a neuronal fate in _Sox10lacZ/Sox10lacZ_mice. (c,h) Immunohistological analysis with antibodies against TuJ-1 (green) and B-FABP (red), which visualize neuronal and glial cells, respectively. Histological appearance of dorsal root ganglia (d,i) and intercostal nerves (e,j) at E12.5. Note the marked reduction in size of the ganglion in the mutant mice. In control mice, nuclei associated with the intercostal nerve are abundant (arrows in e), which are not observed in the Sox10 mutant. Note also the abnormal dorsal root entry zones in homozygous mutants, which are indicated by arrowheads in f, h, and i. Bars (a,f) 80 μm; (b,g) 15 μm; (c,d,h,i) 150 μm; (e,j) 50 μm.
Figure 6
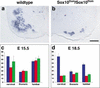
Loss of motoneurons in Sox10Dom/Sox10Dom and _ErbB3_−/− mutant mice. Sections of the cervical spinal cord of wild-type (a) and Sox10Dom/Sox10Dom (b) mutant animals at E18.5 after in situ hybridization with a VAChT specific probe. (c,d) Motoneuron numbers in cervical, thoracic, and lumbar segments of spinal cords obtained from wild-type (blue columns), Sox10Dom/Sox10Dom (red columns), and _ErbB3_−/− (green columns) mutants at E15.5 (c) and E18.5 (d). Mean numbers of motoneurons per section +/− S.D. are shown (n = 4 embryos for each genotype and developmental stage). Counts were not corrected for neuron size, and the numbers of neurons at different developmental stages are therefore not strictly comparable. Bar (a,b) 100 μm.
Figure 7
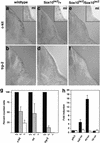
Sox10 controls development of melanoblasts and expression of the trp-2 gene. (a–f) Transverse sections of wild-type (a,b), heterozygous (c,d), and homozygous (e,f) Sox10lacZ mice at E12.5 on corresponding hindlimb levels. Embryos were hybridized with probes specific for c-kit (a,c,e; main panels), mi (a,c,e; insets), and trp-2 (b,d,f). (g) Melanoblasts positive for c-kit, mi, or trp-2 in wild-type (+/+, black), heterozygous (+/−, gray), and homozygous (−/−, white) _Sox10_-deficient mice at E12.5. The numbers of positive cells in wild-type mice identified by each marker were arbitrarily set to 100%. (h) Luciferase reporter assay determining the interaction of Sox10 and the trp-2 gene. Reporter genes were transfected into tet-on N2A cells. Luciferase was expressed under the control of the 3.7-kb trp-2 promoter (trp2luc), the P0 promoter (P0luc), or the thymidine kinase promoter (TKluc) or were lacking a specific promoter (pGL2). Luciferase activity was measured before and after doxycycline treatment. Sox10-dependent promoter activation was expressed as the ratio between these two values. Bars represent the result from three independent transfections +/− S.E.M.
Similar articles
- Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling.
Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Paratore C, et al. Development. 2001 Oct;128(20):3949-61. doi: 10.1242/dev.128.20.3949. Development. 2001. PMID: 11641219 - Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model.
Southard-Smith EM, Kos L, Pavan WJ. Southard-Smith EM, et al. Nat Genet. 1998 Jan;18(1):60-4. doi: 10.1038/ng0198-60. Nat Genet. 1998. PMID: 9425902 - The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia.
Mollaaghababa R, Pavan WJ. Mollaaghababa R, et al. Oncogene. 2003 May 19;22(20):3024-34. doi: 10.1038/sj.onc.1206442. Oncogene. 2003. PMID: 12789277 Review. - A Sox10 expression screen identifies an amino acid essential for Erbb3 function.
Buac K, Watkins-Chow DE, Loftus SK, Larson DM, Incao A, Gibney G, Pavan WJ. Buac K, et al. PLoS Genet. 2008 Sep 5;4(9):e1000177. doi: 10.1371/journal.pgen.1000177. PLoS Genet. 2008. PMID: 18773073 Free PMC article. - Sorting out Sox10 functions in neural crest development.
Kelsh RN. Kelsh RN. Bioessays. 2006 Aug;28(8):788-98. doi: 10.1002/bies.20445. Bioessays. 2006. PMID: 16927299 Review.
Cited by
- Comprehensive analysis of NRG1 common and rare variants in Hirschsprung patients.
Luzón-Toro B, Torroglosa A, Núñez-Torres R, Enguix-Riego MV, Fernández RM, de Agustín JC, Antiñolo G, Borrego S. Luzón-Toro B, et al. PLoS One. 2012;7(5):e36524. doi: 10.1371/journal.pone.0036524. Epub 2012 May 4. PLoS One. 2012. PMID: 22574178 Free PMC article. - Nageotte nodules in human DRG reveal neurodegeneration in painful diabetic neuropathy.
Shiers SI, Mazhar K, Wangzhou A, Haberberger R, Lesnak JB, Sankaranarayanan I, Tavares-Ferreira D, Cervantes A, Funk G, Horton P, Vines E, Dussor G, Price TJ. Shiers SI, et al. bioRxiv [Preprint]. 2024 Aug 23:2024.08.22.609215. doi: 10.1101/2024.08.22.609215. bioRxiv. 2024. PMID: 39229145 Free PMC article. Preprint. - DNA hypomethylation within specific transposable element families associates with tissue-specific enhancer landscape.
Xie M, Hong C, Zhang B, Lowdon RF, Xing X, Li D, Zhou X, Lee HJ, Maire CL, Ligon KL, Gascard P, Sigaroudinia M, Tlsty TD, Kadlecek T, Weiss A, O'Geen H, Farnham PJ, Madden PA, Mungall AJ, Tam A, Kamoh B, Cho S, Moore R, Hirst M, Marra MA, Costello JF, Wang T. Xie M, et al. Nat Genet. 2013 Jul;45(7):836-41. doi: 10.1038/ng.2649. Epub 2013 May 26. Nat Genet. 2013. PMID: 23708189 Free PMC article. - Development and Characterisation of an in vitro Model of Wallerian Degeneration.
Elsayed H, Faroni A, Ashraf MR, Osuji J, Wunderley L, Zhang L, Elsobky H, Mansour M, Zidan AS, Reid AJ. Elsayed H, et al. Front Bioeng Biotechnol. 2020 Jul 10;8:784. doi: 10.3389/fbioe.2020.00784. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32754584 Free PMC article. - Signals that determine Schwann cell identity.
Jessen KR, Mirsky R. Jessen KR, et al. J Anat. 2002 Apr;200(4):367-76. doi: 10.1046/j.1469-7580.2002.00046.x. J Anat. 2002. PMID: 12090403 Free PMC article. Review.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. - PubMed
- Bondurand N, Kuhlbrodt K, Pingault V, Enderich J, Sajus M, Tommerup N, Warburg M, Hennekam RC, Read AP, Wegner M, et al. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum Mol Genet. 1999;8:1785–1789. - PubMed
- Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Caignec CL, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000;9:1907–1917. - PubMed
- Budd PS, Jackson IJ. Structure of the mouse tyrosinase-related protein-2/dopachrome tautomerase (Tyrp2/Dct) gene and sequence of two novel slaty alleles. Genomics. 1995;29:35–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials