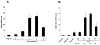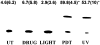Activation of the IL-10 gene promoter following photodynamic therapy of murine keratinocytes - PubMed (original) (raw)
Activation of the IL-10 gene promoter following photodynamic therapy of murine keratinocytes
S O Gollnick et al. Photochem Photobiol. 2001 Feb.
Abstract
Photodynamic therapy (PDT), an anticancer treatment modality, has recently been shown to be an effective treatment for several autoimmune disease models including antigen-induced arthritis. PDT was found to induce the expression of IL-10 messenger RNA (mRNA) and protein in the skin, and this expression has similar kinetics to the appearance of PDT-induced suppression of skin-mediated immune responses such as the contract hypersensitivity (CHS) response. Some aspects of the UVB-induced suppression of the immune response have been linked to the induction of IL-10. IL-10 has been shown to inhibit the development and activation of Th1 cells, which are critical for many cell-mediated immune responses, including CHS. We have examined the effect of PDT and UVB irradiation on the activity of the IL-10 gene promoter and on IL-10 mRNA stability using the murine keratinocyte line, PAM 212. In vitro PDT induces IL-10 mRNA and protein expression from PAM 212 cells, which can be correlated with an increase in AP-1 DNA binding activity and activation of the IL-10 gene promoter by PDT. Deletion of an AP-1 response element from the IL-10 gene promoter was shown to abrogate the PDT-induced promoter activity indicating that the AP-1 response element is critical to IL-10 induction by PDT. In addition, PDT results in an increase in IL-10 mRNA stability, which may also contribute to the increased IL-10 expression in PAM 212 cells following PDT. In vitro UVB irradiation also results in activation of the IL-10 promoter. However, in contrast to PDT, UVB-induced activation of the IL-10 promoter is not AP-1 dependent and did not increase IL-10 mRNA stability.
Figures
Figure 1
Induction of IL-10 mRNA expression in PAM 212 cells by PDT. (A) PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.4 J/cm2 (~LD20). Total RNA was isolated from cells harvested immediately or at 1, 3, 6 and 24 h post-PDT, and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Drug only controls were exposed to Photofrin® and were harvested after 6 h. Results are presented as in relative IL-10 mRNA levels ± SEM following normalization to the actin mRNA levels and represent the results of three independent experiments. (B) PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.2–0.8 J/cm2. Total RNA was isolated from cells harvested 4 h post-PDT and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Drug only and light only controls were exposed to Photofrin® or 0.8 J/cm2 light only, respectively. Results are presented as in relative IL-10 mRNA levels ± SEM as described in “Materials and Methods” and represent the results of three independent experiments. The LD levels corresponding to each light dose are shown below the J/cm2 dose levels.
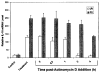
Figure 2
Induction of IL-10 secretion from PAM 212 cells by PDT. PAM 212 cells were treated in vitro, with 2.5 μg/mL Photofrin® in complete medium for 24 h, followed by 3 h drug efflux in complete medium and illumination at 630 nm with 0.4 J/cm2 (~LD20). Immediately following PDT, complete medium was replaced by serum-free medium and the cells were incubated at 37°C in 5%CO2. Supernatants were harvested at 6, 24, 48 and 72 h post-PDT, cells were removed by centrifugation and the supernatants were concentrated 10-fold and the amount of IL-10 present was determined by ELISA as described in “Materials and Methods.” Drug only controls were exposed to Photofrin® and harvested at the same time as the treated cells. Results are reported as the mean ± SEM ng of IL-10 per microgram of total protein.
Figure 3
PDT induces IL-10 gene promoter activity. PAM 212 cells were transiently transfected with pIL10pro(−1626)CAT followed by either PDT or UVB treatment as described in “Materials and Methods.” Cells were harvested 48 h after treatment and CAT assays were performed with 100 μg of cell lysates. A representative experiment is shown. The numbers above each lane reflect the results [normalized percent conversion ± (SEM)] of a minimum of three independent experiments. Controls are represented as transfected untreated cells (UT) and transfected cells subjected to either light alone (light) or Photofrin® alone (drug). *Significant compared to controls.
Figure 4
Identification of a 593 bp region of the IL-10 promoter required for PDT induction. (A) PAM 212 cells were transiently transfected with 5′-deletion constructs of the IL-10 promoter (pIL10pro(−1033)CAT, pIL10pro(−885)CAT, pIL10pro(−863)CAT, pIL10pro(−125)CAT), the full length promoter pIL10pro(−1626)CAT construct or the pCAT3basic vector, followed by PDT or UVB irradiation. CAT assays were performed as above. Results are presented as normalized mean percent conversion of chloramphenicol to acetylated chloramphenicol ± SEM.
Figure 5
PDT induces AP-1 DNA binding activity in PAM 212 cells. (A) Nuclear extracts were isolated from PAM 212 cells at 0, 0.5, 3, 6, 24 h post-PDT and subjected to EMSA with radiolabeled AP-1, oligonucleotide as described in “Materials and Methods.” Nuclear extracts isolated from untreated cells (UT) and cells treated with Photofrin® (D) alone were used as controls. Free probe is also shown (F). Results from a representative experiment are shown. Extracts isolated from three independent experiments showed similar results. An arrow indicates the AP-1 complex. (B) Nuclear extracts were isolated 3 h post-PDT treatment were subjected to EMSA with radiolabeled AP-1 oligonucleotide in the presence or absence of 100 or 500-fold excess cold oligonucleotides to test for protein:DNA complex specificity. (C) Nuclear extracts were isolated 3 h post-PDT treatment and subjected to EMSA with radiolabeled AP-1 oligonucleotide in the presence or absence of 4 μg of anti-c-fos, anti-c-jun/AP-1 or control rabbit IgG, C, to identify proteins within the protein: DNA complex. The AP-1 and supershifted (ss) complexes are indicated with arrows.
Figure 6
Deletion of the AP-1 response element from the IL-10 promoter abrogates the PDT responsiveness. PAM 212 cells were: (A) transiently transfected with pIL10(−1626)CAT, pIL10(ΔAP1)CAT or pCAT3basic followed by PDT; or (B) UVB irradiation as described above. CAT assays were done as described in “Materials and Methods.” A representative experiment is shown; the numbers above each lane reflect the results [normalized percent conversion (SEM)] of a minimum of three independent experiments.
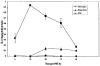
Figure 7
PDT prolongs IL-10 mRNA half-life. Actinomycin D (10 μg/mL) was added to PAM 212 cells 1 or 4 h following UVB irradiation or PDT treatment, respectively. Total RNA was isolated from cells harvested immediately (0) or at 1, 2 or 4 h post-actinomycin D addition and 2 μg from each sample were subjected to RT-PCR analysis as described in “Materials and Methods.” Results are presented as in relative IL-10 mRNA levels ± SEM following normalization to the actin mRNA levels. Control values refer to untreated controls for the UVB sample and Photofrin® treatment only for the PDT sample. Controls and treatment samples were harvested at 1 h for the UVB samples and 4 h for the PDT samples.
Similar articles
- Relative contribution of NF-kappaB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by keratinocytes.
Grandjean-Laquerriere A, Gangloff SC, Le Naour R, Trentesaux C, Hornebeck W, Guenounou M. Grandjean-Laquerriere A, et al. Cytokine. 2002 May 7;18(3):168-77. doi: 10.1006/cyto.2002.0888. Cytokine. 2002. PMID: 12126654 - Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kappa B DNA binding.
Kick G, Messer G, Goetz A, Plewig G, Kind P. Kick G, et al. Cancer Res. 1995 Jun 1;55(11):2373-9. Cancer Res. 1995. PMID: 7757989 - IL-10 does not play a role in cutaneous Photofrin photodynamic therapy-induced suppression of the contact hypersensitivity response.
Gollnick SO, Musser DA, Oseroff AR, Vaughan L, Owczarczak B, Henderson BW. Gollnick SO, et al. Photochem Photobiol. 2001 Dec;74(6):811-6. doi: 10.1562/0031-8655(2001)074<0811:idnpar>2.0.co;2. Photochem Photobiol. 2001. PMID: 11783937 - TNF-alpha production in the skin.
Bashir MM, Sharma MR, Werth VP. Bashir MM, et al. Arch Dermatol Res. 2009 Jan;301(1):87-91. doi: 10.1007/s00403-008-0893-7. Epub 2008 Sep 30. Arch Dermatol Res. 2009. PMID: 18825399 Review. - The immunosuppressive side of PDT.
Mroz P, Hamblin MR. Mroz P, et al. Photochem Photobiol Sci. 2011 May;10(5):751-8. doi: 10.1039/c0pp00345j. Epub 2011 Mar 24. Photochem Photobiol Sci. 2011. PMID: 21437314 Free PMC article. Review.
Cited by
- Bibliometric analysis of photodynamic therapy and immune response from 1989-2023.
Fan W, Tang J, Tang S, Lin Z, Li M, Zhang Z, Wu D. Fan W, et al. Front Pharmacol. 2024 Jan 15;15:1299253. doi: 10.3389/fphar.2024.1299253. eCollection 2024. Front Pharmacol. 2024. PMID: 38288443 Free PMC article. - IL-10 in glioma.
Widodo SS, Dinevska M, Furst LM, Stylli SS, Mantamadiotis T. Widodo SS, et al. Br J Cancer. 2021 Nov;125(11):1466-1476. doi: 10.1038/s41416-021-01515-6. Epub 2021 Aug 4. Br J Cancer. 2021. PMID: 34349251 Free PMC article. Review. - Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens.
Zhang T, Ying D, Qi M, Li X, Fu L, Sun X, Wang L, Zhou Y. Zhang T, et al. Molecules. 2019 Jul 24;24(15):2692. doi: 10.3390/molecules24152692. Molecules. 2019. PMID: 31344909 Free PMC article. - Photodynamic activation as a molecular switch to promote osteoblast cell differentiation via AP-1 activation.
Kushibiki T, Tu Y, Abu-Yousif AO, Hasan T. Kushibiki T, et al. Sci Rep. 2015 Aug 17;5:13114. doi: 10.1038/srep13114. Sci Rep. 2015. PMID: 26279470 Free PMC article. - Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death.
Castano AP, Demidova TN, Hamblin MR. Castano AP, et al. Photodiagnosis Photodyn Ther. 2005 Mar;2(1):1-23. doi: 10.1016/S1572-1000(05)00030-X. Photodiagnosis Photodyn Ther. 2005. PMID: 25048553 Free PMC article.
References
- Ratkay LG, Chowdhary RK, Iamaroon A, Richter AM, Neyndorff HC, Keystone EC, Waterfield JD, Levy JG. Amelioration of antigen-induced arthritis in rabbits by induction of apoptosis of inflammatory cells with local application of transdermal photodynamic therapy. Arthritis Rheum. 1998;41:525–534. - PubMed
- Trauner K, Gandouredwards R, Bamberf M, Nishioka NS, Flotte T, Autry S, Hasan T. Influence of light delivery on photodynamic synovectomy in an antigen-induced arthritis model for rheumatoid arthritis. Lasers Surg Med. 1998;22:147–156. - PubMed
- Leong S, Chan AH, Levy JG, Hunt DWC. Transcutaneous photodynamic therapy alters the development of an adoptively transferred form of murine experimentall autoimmune encephalomyelitis. Photochem Photobiol. 1996;64:751–757. - PubMed
- Boehncke WH, Elshorst-Schmidt T, Kaufmann R. Systemic photodynamic therapy is a safe and effective treatment for psoriasis. Arch Dermatol. 2000;136:271–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016056/CA/NCI NIH HHS/United States
- P01 CA055791-120002/CA/NCI NIH HHS/United States
- P01 CA055791/CA/NCI NIH HHS/United States
- P30 CA16056/CA/NCI NIH HHS/United States
- P01 CA055791-110002/CA/NCI NIH HHS/United States
- P01 CA55791/CA/NCI NIH HHS/United States
- P01 CA055791-130002/CA/NCI NIH HHS/United States
- P01 CA055791-140002/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous