Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen - PubMed (original) (raw)
Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen
J Hernandez et al. J Exp Med. 2001.
Abstract
Not all T cells specific for autoantigens are eliminated in the thymus, and therefore alternate mechanisms are required to prevent potentially autoreactive T cells from developing into effectors. Adoptive transfer of CD8(+) T cells from influenza hemagglutinin-specific Clone 4 TCR transgenic mice into mice that express hemagluttinin in the pancreatic islets results in tolerance. This is preceded by activation of Clone 4 T cells that encounter antigen cross-presented in the draining lymph nodes of the pancreas. In this report we compare the phenotype, function, and costimulatory requirements of Clone 4 T cells activated by endogenous self-antigen, with Clone 4 T cells stimulated by influenza virus. The cells undergoing tolerance upregulate both CD69 and CD44, yet only partially downregulate CD62L, and do not express CD49d or CD25. Most importantly, they lack the ability to produce interferon-gamma in response to antigen and show no cytolytic activity. Clone 4 T cells disappear after several cycles of division, apparently without leaving the site of initial activation. Surprisingly, despite the fact that such stimulation occurs through recognition of antigen that is cross-presented by a professional antigen-presenting cell, we find this activation is not dependent on costimulation through CD28. These data demonstrate that the recognition by naive CD8(+) T cells of cross-presented self-antigen results in localized proliferation and deletion, without the production of effector cells.
Figures
Figure 1
Clone 4 CD8+ T cells proliferate in the pancreatic LNs of InsHA mice but do not accumulate over time. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into either Balb/c or InsHA hosts. Injected InsHA mice were split into two groups, one was immunized with influenza virus at the time of transfer and the other was left untreated. Mice were killed on days 4 or 8 after transfer and cells from pooled LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on CD8+ Thy1.1+ lymphocytes. This experiment has been repeated five times for InsHA mice and three times for influenza immunized InsHA and Balb/c mice. (B) Total numbers of CD8+ Thy1.1+ cells in the pancreatic LNs of host mice. Data represent the mean of all independent experiments performed. Only negative standard deviation is depicted to achieve greater sensitivity in the graph.
Figure 1
Clone 4 CD8+ T cells proliferate in the pancreatic LNs of InsHA mice but do not accumulate over time. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into either Balb/c or InsHA hosts. Injected InsHA mice were split into two groups, one was immunized with influenza virus at the time of transfer and the other was left untreated. Mice were killed on days 4 or 8 after transfer and cells from pooled LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on CD8+ Thy1.1+ lymphocytes. This experiment has been repeated five times for InsHA mice and three times for influenza immunized InsHA and Balb/c mice. (B) Total numbers of CD8+ Thy1.1+ cells in the pancreatic LNs of host mice. Data represent the mean of all independent experiments performed. Only negative standard deviation is depicted to achieve greater sensitivity in the graph.
Figure 2
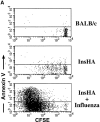
Detection of apoptotic Clone 4 CD8+ T cells. CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into the indicated hosts as in Fig. 1. On day 4 after transfer mice were killed and cells from pooled pancreatic LNs were analyzed by FACS® to detect apoptotic cells through annexin V binding. (A) Plots represent the amount of CFSE label versus annexin V binding–intensity gating on lymphocytes CD8+ Thy1.1+. One representative experiment out three independent experiments is shown. (B) Percentage of Clone 4 Thy1.1+ CD8+ T cells that are annexin V+ in the pancreatic LNs of recipient mice. Data represent the mean of three independent experiments performed.
Figure 2
Detection of apoptotic Clone 4 CD8+ T cells. CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into the indicated hosts as in Fig. 1. On day 4 after transfer mice were killed and cells from pooled pancreatic LNs were analyzed by FACS® to detect apoptotic cells through annexin V binding. (A) Plots represent the amount of CFSE label versus annexin V binding–intensity gating on lymphocytes CD8+ Thy1.1+. One representative experiment out three independent experiments is shown. (B) Percentage of Clone 4 Thy1.1+ CD8+ T cells that are annexin V+ in the pancreatic LNs of recipient mice. Data represent the mean of three independent experiments performed.
Figure 3
Phenotypic characterization of proliferating Clone 4 CD8+ T cells. CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into different hosts as in Fig. 1. On day 4 after transfer mice were killed and cells from pooled pancreatic LNs were analyzed by FACS® to detect expression of key activation surface markers. (A) Plots represent the amount of CFSE label versus the intensity of expression of five different activation markers gating on lymphocytes CD8+ Thy1.1+. One out of two independent experiments with similar results is shown. (B) Representation of the geometrical mean fluorescence intensity (GMFI) of the different activation markers from A examined as a function of the division cycles. GMFI was selected for this analysis due to the small numbers of cells involved in the analysis of each cycle of division, particularly in the case of Clone 4 T cells activated by endogenous expression of HA.
Figure 4
IFN-γ production by proliferating Clone 4 CD8+ T cells. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or left untreated. On day 4 after transfer cells from pooled pancreatic LNs were incubated with Kd HA peptide (stimulated) or an irrelevant peptide (nonstimulated) for 6 h at 37°C. Then, cells were analyzed by FACS® to detect accumulation of intracellular IFN-γ. (A) Plots represent the amount of CFSE label versus the intensity of IFN-γ produced gating on lymphocytes CD8+ Thy1.1+. Data correspond to one out of two independent experiments with similar results. (B) Representation of the percentage of CD8+ Thy1.1+ T cells that are IFN-γ+ from A as a function of the division cycle.
Figure 4
IFN-γ production by proliferating Clone 4 CD8+ T cells. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or left untreated. On day 4 after transfer cells from pooled pancreatic LNs were incubated with Kd HA peptide (stimulated) or an irrelevant peptide (nonstimulated) for 6 h at 37°C. Then, cells were analyzed by FACS® to detect accumulation of intracellular IFN-γ. (A) Plots represent the amount of CFSE label versus the intensity of IFN-γ produced gating on lymphocytes CD8+ Thy1.1+. Data correspond to one out of two independent experiments with similar results. (B) Representation of the percentage of CD8+ Thy1.1+ T cells that are IFN-γ+ from A as a function of the division cycle.
Figure 5
In vivo cytolytic activity of proliferating Clone 4 CD8+ T cells. 3 × 106 purified Clone 4 CD8+ T cells were injected into either Balb/c or InsHA hosts. Injected InsHA mice were split into two groups, one was immunized with influenza virus at the time of transfer and the other was left untreated. 4 d after transfer mice were injected with a mixture of 2.5 × 106 CFSEhigh-labeled, Kd HA peptide pulsed syngenic spleen cells, and 2.5 × 106 CFSElow-labeled, nonpulsed cells, as targets. Groups of mice that did not receive Clone 4 cells were used as controls and were also given CFSE-labeled target cells. 10 h later lymphocytes from the pancreatic LNs of four individual mice per group (n = 4) were examined by FACS® to detect and quantify CFSE-labeled cells. Histograms represent the amount of CFSE label of one representative mouse per group. The mean ± SD of the ratio of the number of CFSElow/CFSEhigh cells (r) for all mice in each group is indicated. Data represent one out of two experiments with similar results.
Figure 6
IFN-γ production by proliferating Clone 4 CD8+ T cells is not dependent on the antigen dose. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into Balb/c recipients. Cells recovered from the pancreatic LNs were analyzed for their ability to produce IFN-γ as described in Fig. 4. (A) Mice were immunized with 12 HA U of influenza virus at the time of cell transfer and killed on day 4. (B) Mice were immunized with 100 μg of Kd HA peptide intravenously at the time of cell transfer and killed on day 3.
Figure 7
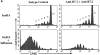
Proliferation of Clone 4 cells in the pancreatic LNs of InsHA mice is B7 co-stimulation independent. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or not. Half of the mice from the two previous groups were treated anti-B7.1 plus anti-B7.2 mAbs and the rest of the mice were treated with rat plus hamster IgG as isotype controls. On day 4 after transfer, cells from the pancreatic LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on lymphocytes CD8+ Thy1.1+. Percentages of cells within each division cycle are indicated in the top histograms. Data correspond to one out of three independent experiments with similar results. (B) Total numbers of CD8+ Thy1.1+ T cells in the pancreatic LNs of recipient mice. Data represents the mean of all experiments performed. (C) Cells from the pancreatic LNs were processed as described in Fig. 4 to detect IFN-γ production. The percentage of lymphocytes CD8+ Thy1.1+ that are IFN-γ+ is represented.
Figure 7
Proliferation of Clone 4 cells in the pancreatic LNs of InsHA mice is B7 co-stimulation independent. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or not. Half of the mice from the two previous groups were treated anti-B7.1 plus anti-B7.2 mAbs and the rest of the mice were treated with rat plus hamster IgG as isotype controls. On day 4 after transfer, cells from the pancreatic LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on lymphocytes CD8+ Thy1.1+. Percentages of cells within each division cycle are indicated in the top histograms. Data correspond to one out of three independent experiments with similar results. (B) Total numbers of CD8+ Thy1.1+ T cells in the pancreatic LNs of recipient mice. Data represents the mean of all experiments performed. (C) Cells from the pancreatic LNs were processed as described in Fig. 4 to detect IFN-γ production. The percentage of lymphocytes CD8+ Thy1.1+ that are IFN-γ+ is represented.
Figure 7
Proliferation of Clone 4 cells in the pancreatic LNs of InsHA mice is B7 co-stimulation independent. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or not. Half of the mice from the two previous groups were treated anti-B7.1 plus anti-B7.2 mAbs and the rest of the mice were treated with rat plus hamster IgG as isotype controls. On day 4 after transfer, cells from the pancreatic LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on lymphocytes CD8+ Thy1.1+. Percentages of cells within each division cycle are indicated in the top histograms. Data correspond to one out of three independent experiments with similar results. (B) Total numbers of CD8+ Thy1.1+ T cells in the pancreatic LNs of recipient mice. Data represents the mean of all experiments performed. (C) Cells from the pancreatic LNs were processed as described in Fig. 4 to detect IFN-γ production. The percentage of lymphocytes CD8+ Thy1.1+ that are IFN-γ+ is represented.
Similar articles
- Perinatal blockade of b7-1 and b7-2 inhibits clonal deletion of highly pathogenic autoreactive T cells.
Gao JX, Zhang H, Bai XF, Wen J, Zheng X, Liu J, Zheng P, Liu Y. Gao JX, et al. J Exp Med. 2002 Apr 15;195(8):959-71. doi: 10.1084/jem.20011948. J Exp Med. 2002. PMID: 11956287 Free PMC article. - B7-2 (CD86) controls the priming of autoreactive CD4 T cell response against pancreatic islets.
Yadav D, Judkowski V, Flodstrom-Tullberg M, Sterling L, Redmond WL, Sherman L, Sarvetnick N. Yadav D, et al. J Immunol. 2004 Sep 15;173(6):3631-9. doi: 10.4049/jimmunol.173.6.3631. J Immunol. 2004. PMID: 15356107 - Abortive activation precedes functional deletion of CD8+ T cells following encounter with self-antigens expressed by resting B cells in vivo.
Fraser JM, Janicki CN, Raveney BJ, Morgan DJ. Fraser JM, et al. Immunology. 2006 Sep;119(1):126-33. doi: 10.1111/j.1365-2567.2006.02414.x. Epub 2006 Jun 23. Immunology. 2006. PMID: 16796693 Free PMC article. - Cross-presentation of self antigens to CD8+ T cells: the balance between tolerance and autoimmunity.
Kurts C, Heath WR, Carbone FR, Kosaka H, Miller JF. Kurts C, et al. Novartis Found Symp. 1998;215:172-81; discussion 181-90. doi: 10.1002/9780470515525.ch13. Novartis Found Symp. 1998. PMID: 9760579 Review. - Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens.
Miller JF, Kurts C, Allison J, Kosaka H, Carbone F, Heath WR. Miller JF, et al. Immunol Rev. 1998 Oct;165:267-77. doi: 10.1111/j.1600-065x.1998.tb01244.x. Immunol Rev. 1998. PMID: 9850866 Review.
Cited by
- The danger theory of immunity revisited.
Kroemer G, Montégut L, Kepp O, Zitvogel L. Kroemer G, et al. Nat Rev Immunol. 2024 Nov 7. doi: 10.1038/s41577-024-01102-9. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39511426 Review. - CMTM6 shapes antitumor T cell response through modulating protein expression of CD58 and PD-L1.
Miao B, Hu Z, Mezzadra R, Hoeijmakers L, Fauster A, Du S, Yang Z, Sator-Schmitt M, Engel H, Li X, Broderick C, Jin G, Gomez-Eerland R, Rozeman L, Lei X, Matsuo H, Yang C, Hofland I, Peters D, Broeks A, Laport E, Fitz A, Zhao X, Mahmoud MAA, Ma X, Sander S, Liu HK, Cui G, Gan Y, Wu W, Xiao Y, Heck AJR, Guan W, Lowe SW, Horlings HM, Wang C, Brummelkamp TR, Blank CU, Schumacher TNM, Sun C. Miao B, et al. Cancer Cell. 2023 Oct 9;41(10):1817-1828.e9. doi: 10.1016/j.ccell.2023.08.008. Epub 2023 Sep 7. Cancer Cell. 2023. PMID: 37683639 Free PMC article. - Pancreatic draining lymph nodes (PLNs) serve as a pathogenic hub contributing to the development of type 1 diabetes.
Sun F, Yang CL, Wang FX, Rong SJ, Luo JH, Lu WY, Yue TT, Wang CY, Liu SW. Sun F, et al. Cell Biosci. 2023 Aug 28;13(1):156. doi: 10.1186/s13578-023-01110-7. Cell Biosci. 2023. PMID: 37641145 Free PMC article. Review. - Current Concepts of Vitiligo Immunopathogenesis.
Hlača N, Žagar T, Kaštelan M, Brajac I, Prpić-Massari L. Hlača N, et al. Biomedicines. 2022 Jul 8;10(7):1639. doi: 10.3390/biomedicines10071639. Biomedicines. 2022. PMID: 35884944 Free PMC article. Review. - Variegated Outcomes of T Cell Activation by Dendritic Cells in the Steady State.
Bourque J, Hawiger D. Bourque J, et al. J Immunol. 2022 Feb 1;208(3):539-547. doi: 10.4049/jimmunol.2100932. J Immunol. 2022. PMID: 35042789 Free PMC article. Review.
References
- Kappler J.W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. - PubMed
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., Boehmer H.V. Tolerance in T cell receptor transgenic mice involves deletion of non mature CD4+8− thymocytes. Nature. 1988;333:742–746. - PubMed
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. - PMC - PubMed
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous