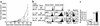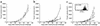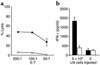T cell homeostatic proliferation elicits effective antitumor autoimmunity - PubMed (original) (raw)
T cell homeostatic proliferation elicits effective antitumor autoimmunity
Wolfgang Dummer et al. J Clin Invest. 2002 Jul.
Abstract
Development of tumor immunotherapies focuses on inducing autoimmune responses against tumor-associated self-antigens primarily encoded by normal, unmutated genes. We hypothesized that such responses could be elicited by T cell homeostatic proliferation in the periphery, involving expansion of T cells recognizing self-MHC/peptide ligands. Herein, we demonstrate that sublethally irradiated lymphopenic mice transfused with autologous or syngeneic T cells showed tumor growth inhibition when challenged with melanoma or colon carcinoma cells. Importantly, the antitumor response depended on homeostatic expansion of a polyclonal T cell population within lymph nodes. This response was effective even for established tumors, was characterized by CD8(+) T cell-mediated tumor-specific cytotoxicity and IFN-gamma production, and was associated with long-term memory. The results indicate that concomitant induction of the physiologic processes of homeostatic T cell proliferation and tumor antigen presentation in lymph nodes triggers a beneficial antitumor autoimmune response.
Figures
Figure 1
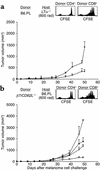
Inhibition of melanoma growth by homeostatic T cell proliferation. (a) Nonirradiated (circles) or sublethally irradiated (squares) C57BL/6 mice were challenged subcutaneously with 5 × 105 B78D14 melanoma cells. Groups of irradiated mice were transfused with 5 × 106 (inverted triangles) or 5 × 107 (triangles) syngeneic B6.PL LN cells. Means and standard deviation of tumor growth are indicated (all groups, n = 7). (b) Proliferation profiles of gated Thy1.1+CD4+ and Thy1.1+CD4– (CD8+) cells in secondary lymphoid organs shown as CFSE histograms. Upper panel: nonirradiated C57BL/6 mice (Thy1.2+) transfused with 107 B6.PL (Thy1.1+) cells; middle panel: C57BL/6 mice irradiated and transfused with 5 × 106 B6.PL LN cells; lower panel: C57BL/6 mice irradiated and transfused with 5 × 107 B6.PL LN cells. FACS dot plots of host lymphoid organs are depicted to the right. (c) Total number of proliferating (one or more cell divisions) donor CD4+ and CD8+ cells in C57BL/6 recipients of 5 × 106 or 5 × 107 B6.PL LN cells calculated from data presented in b.
Figure 2
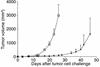
Polyclonal homeostatic expansion of CD8+ T cells is sufficient for melanoma growth inhibition. (a) Nonirradiated (circles) and irradiated (triangles) B6.RAG–/– mice challenged with melanoma cells show no difference in tumor growth (n = 5 for each group). (b) Transfusion of 5 × 106 syngeneic B6.LN cells to nonirradiated B6.RAG–/– mice leads to significant melanoma growth inhibition (triangles) compared with nonirradiated, nontransfused controls (circles). Adoptive transfer of purified C57BL/6 CD8+ T cells is sufficient to exert an antitumor effect (squares) equal to that of whole LN cells (all groups, n = 7). (c) Adoptive transfer of 5 × 106 2C CD8+ TCR transgenic cells into irradiated B6 hosts (triangles) did not enhance the antitumor effect achieved by irradiation alone (squares), despite the ability of 2C cells to undergo homeostatic proliferation (inset). Controls included nonirradiated, nontransfused C57BL/6 mice (circles) and irradiated, transfused (5 × 106 B6 LN cells) C57BL/6 mice (inverted triangles) (all groups, n = 6).
Figure 3
Specific recognition of tumors induced by homeostatic proliferation. (a) Sublethally irradiated C57BL/6 mice were transfused with 5 × 106 LN cells prior to challenge with B78D14 melanoma cells (n = 4). Ten days after challenge, splenocytes were coincubated with either irradiated B78D14 melanoma cells (filled squares) or irradiated MC-38 colon carcinoma cells (open squares) for 48 hours; a standard 4-hour 51Cr-release assay was performed at three effector-to-target cell ratios. Results indicate percentage of specific lysis from a pool of four mice assessed in triplicate. (b) Sublethally irradiated C57BL/6 mice were transfused with 5 × 106 LN cells prior to challenge with B78D14 melanoma cells. Thirty-five days after challenge, splenocytes were cultured for 72 hours with either B78D14 melanoma cells (black bars) or MC-38 colon carcinoma cells (white bars). Similarly cultured control lymphocytes were derived from nonirradiated, nontransfused, and melanoma-challenged mice. Results indicate IFN-γ levels in the supernatants from a pool of four mice assessed in quadruplicate.
Figure 4
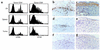
Proliferation and immunohistochemistry of tumor-infiltrating T cells. (a) Groups of C57BL/6 mice (Thy1.2+) were sublethally irradiated, challenged with melanoma cells, and transfused with 5 × 106 CFSE-labeled syngeneic B6.PL (Thy1.1+) LN cells. Four weeks later, single cell suspensions of host LN, spleen, and tumor tissue were analyzed for division characteristics by flow cytometry of gated Thy1.1+CD4+ and Thy1.1+CD4– (CD8+) donor cells. (b–g) Groups of C57BL/6 mice were untreated or sublethally irradiated and challenged with melanoma cells. The irradiated mice were then transfused with 5 × 107 syngeneic B6.PL LN cells. Immunohistochemistry of residual tumors in transfused (b, d, f) mice compared with large tumors of untreated controls (c, e, g) is shown at day 60. Staining with an anti-Thy1.1 Ab (OX-7) reveals donor cells in the regressed tumor tissue of the LN cell–transfused irradiated host (b), but not in the progressing melanoma tumor of transfused, nonirradiated animals (c). Staining for CD4+ cells demonstrates considerable infiltration in the irradiated reconstituted group (d), but minor infiltration in the nonirradiated, nonreconstituted mice (e). Similarly, CD8+ cells are found in higher numbers in irradiated and transfused (f) than in nonirradiated, nontransfused (g) mice. Staining for CD56 did not reveal appearance of NK cells (not shown).
Figure 5
T cells need to home to the regional LN to mediate tumor growth inhibition. (a) Melanoma growth inhibition is less efficient in sublethally irradiated, LN cell–transfused LTα-deficient B6 mice (triangles) than in LTα+/+ mice (inverted triangles) (both groups n = 6). Homeostatic proliferation characteristics of CFSE-labeled B6.PL (Thy1.1+) CD4+ and CD8+ T cells in the spleen of an irradiated B6.LTα–/– host is intact, as measured by flow cytometry on day 7 after transfer (inset). (b) Adoptive transfer of β7/CD62L–/– spleen cells into irradiated C57BL/6 mice (triangles) does not lead to enhanced tumor growth inhibition compared with irradiated, nontransfused C57BL/6 controls (squares). Homeostatic proliferation of CFSE-labeled B6.β7/CD62L–/– (Thy1.2+) CD4+ and CD8+ T cells in the spleen of an irradiated B6.PL host (Thy1.1+) is intact, as measured by flow cytometry on day 7 after transfer (inset). Controls included nonirradiated, nontransfused (circles), and irradiated C57BL/6 mice transfused with 5 × 106 B6 LN cells (inverted triangles).
Figure 6
Effect of homeostatic T cell expansion on growth of a colon carcinoma cell line. Sublethally irradiated C57BL/6 mice were challenged subcutaneously with 105 MC-38 colon carcinoma cells, and half of the mice were transfused with 5 × 107 B6 LN cells. Tumor growth was inhibited in the transfused (triangles) compared with the nontransfused (squares) mice (both groups, n = 6).
Comment in
- Making room for T cells.
Maine GN, Mulé JJ. Maine GN, et al. J Clin Invest. 2002 Jul;110(2):157-9. doi: 10.1172/JCI16166. J Clin Invest. 2002. PMID: 12122106 Free PMC article. Review. No abstract available.
Similar articles
- Immunotherapy of melanoma: a dichotomy in the requirement for IFN-gamma in vaccine-induced antitumor immunity versus adoptive immunotherapy.
Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Winter H, et al. J Immunol. 2001 Jun 15;166(12):7370-80. doi: 10.4049/jimmunol.166.12.7370. J Immunol. 2001. PMID: 11390488 - Vaccine therapy of established tumors in the absence of autoimmunity.
Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Hodge JW, et al. Clin Cancer Res. 2003 May;9(5):1837-49. Clin Cancer Res. 2003. PMID: 12738742 - CD30 prevents T-cell responses to non-lymphoid tissues.
Heath WR, Kurts C, Caminschi I, Carbone FR, Miller JF. Heath WR, et al. Immunol Rev. 1999 Jun;169:23-9. doi: 10.1111/j.1600-065x.1999.tb01303.x. Immunol Rev. 1999. PMID: 10450505 Review. - Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives.
Baccala R, Gonzalez-Quintial R, Dummer W, Theofilopoulos AN. Baccala R, et al. Springer Semin Immunopathol. 2005 Jun;27(1):75-85. doi: 10.1007/s00281-004-0196-9. Epub 2005 Jan 22. Springer Semin Immunopathol. 2005. PMID: 15666151 Review.
Cited by
- Mathematical modeling of endogenous and exogenously administered T cell recirculation in mouse and its application to pharmacokinetic studies of cell therapies.
Nikitich A, Helmlinger G, Peskov K, Bocharov G. Nikitich A, et al. Front Immunol. 2024 Apr 17;15:1357706. doi: 10.3389/fimmu.2024.1357706. eCollection 2024. Front Immunol. 2024. PMID: 38846946 Free PMC article. - IL-2-free tumor-infiltrating lymphocyte therapy with PD-1 blockade demonstrates potent efficacy in advanced gynecologic cancer.
Guo J, Wang C, Luo N, Wu Y, Huang W, Zhu J, Shi W, Ding J, Ge Y, Liu C, Lu Z, Bast RC Jr, Ai G, Yang W, Wang R, Li C, Chen R, Liu S, Jin H, Zhao B, Cheng Z. Guo J, et al. BMC Med. 2024 May 20;22(1):207. doi: 10.1186/s12916-024-03420-0. BMC Med. 2024. PMID: 38769543 Free PMC article. - How I treat cytopenias after CAR T-cell therapy.
Jain T, Olson TS, Locke FL. Jain T, et al. Blood. 2023 May 18;141(20):2460-2469. doi: 10.1182/blood.2022017415. Blood. 2023. PMID: 36800563 Free PMC article. - Clinical Strategies for Enhancing the Efficacy of CAR T-Cell Therapy for Hematological Malignancies.
Liu Q, Liu Z, Wan R, Huang W. Liu Q, et al. Cancers (Basel). 2022 Sep 14;14(18):4452. doi: 10.3390/cancers14184452. Cancers (Basel). 2022. PMID: 36139611 Free PMC article. Review. - Lymphopenia and Mechanisms of T-Cell Regeneration.
Saidakova EV. Saidakova EV. Cell tissue biol. 2022;16(4):302-311. doi: 10.1134/S1990519X2204006X. Epub 2022 Aug 8. Cell tissue biol. 2022. PMID: 35967247 Free PMC article.
References
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. - PubMed
- Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol. 1997;9:681–683. - PubMed
- Nanda NK, Sercarz EE. Induction of anti-self-immunity to cure cancer. Cell. 1995;82:13–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR039555/AR/NIAMS NIH HHS/United States
- CA-83856/CA/NCI NIH HHS/United States
- AG-15061/AG/NIA NIH HHS/United States
- AR-39555/AR/NIAMS NIH HHS/United States
- AR-31203/AR/NIAMS NIH HHS/United States
- R01 AR031203/AR/NIAMS NIH HHS/United States
- R37 AR039555/AR/NIAMS NIH HHS/United States
- R01 CA083856/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials