Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study - PubMed (original) (raw)
Clinical Trial
doi: 10.1007/s00134-003-1650-1. Epub 2003 Feb 15.
Thomas Schaible, Claudia Roll, Jan-Holger Schiffmann, Lutz Bindl, Lothar Schrod, Irwin Reiss, Martina Kohl, Subha Demirakca, Roland Hentschel, Thomas Paul, Anne Vierzig, Peter Groneck, Heide von Seefeld, Helmut Schumacher, Ludwig Gortner; Surfactant ARDS Study Group
Affiliations
- PMID: 12589529
- PMCID: PMC7095123
- DOI: 10.1007/s00134-003-1650-1
Clinical Trial
Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study
Jens Christian Möller et al. Intensive Care Med. 2003 Mar.
Abstract
Objective: To determine whether bovine surfactant given in cases of severe pediatric acute respiratory distress syndrome (ARDS) improves oxygenation.
Design: Single-center study with 19 patients, followed by a multicenter randomized comparison of surfactant with a standardized treatment algorithm. Primary endpoint PaO(2)/FIO(2) at 48 h, secondary endpoints: PaO(2)/FIO(2) at 2, 4, 12, and 24 h, survival, survival without rescue, days on ventilator, subgroups analyzed by analysis of variance to identify patients who might benefit from surfactant.
Setting: Multicenter study in 19 reference centers for ARDS.
Patients: Children after the 44th postconceptional week and under 14 years old, admitted for at least 4 h, ventilated for 12-120 h, and without heart failure or chronic lung disease. In the multicenter study 35 patients were recruited; 20 were randomized to the surfactant group and 15 to the nonsurfactant group. Decreasing recruitment of patients led to a preliminary end of this study.
Interventions: Administration of 100 mg/kg bovine surfactant intratracheally under continuous ventilation and PEEP, as soon as the PaO(2)/FIO(2) ratio dropped to less than 100 for 2 h (in the pilot study increments of 50 mg/kg as long as the PaO(2)/FIO(2) did not increase by 20%). A second equivalent dose within 48 h was permitted.
Results: In the pilot study the PaO(2)/FIO(2) increased by a mean of 100 at 48 h (n=19). A higher PaO(2)/FIO(2) ratio was observed in the surfactant group 2 h after the first dose (58 from baseline vs. 9), at 48 h there was a trend towards a higher ratio (38 from baseline vs. 22). The rate of rescue therapy was significantly lower in the surfactant group. Outcome criteria were not affected by a second surfactant dose (n=11). A significant difference in PaO(2)/FIO(2) in favor of surfactant at 48 h was found in the subgroup with an initial PaO(2)/FIO(2) ratio higher than 65 and in patients without pneumonia. CONCLUSIONS. Surfactant therapy in severe ARDS improves oxygenation immediately after administration. This improvement is sustained only in the subgroup of patients without pneumonia and that with an initial PaO(2)/FIO(2) ratio higher than 65
Figures
Fig 1.
Algorithm for ventilatory management in the study centers before and after randomization
Fig. 2.
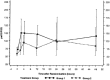
Medians and interquartile ranges (25–75 percentiles) of the oxygenation index (PaO2/FIO2) in the 48-h observation period of the surfactant group (group 1) and controls (group 2)
Fig. 3.
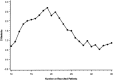
Medians and interquartile ranges (25–75 percentiles) of changes of peak inspiratory pressure in cmH2O (a) and positive end-expiratory pressure (b) over the 48-h observation period in the surfactant group (group 1) and controls (group 2)
Fig. 4.
Z statistic of the primary endpoint, change in PaO2/FIO2 ratio at 48 h between the surfactant group (group 1) and controls (group 2)
Fig. 5.
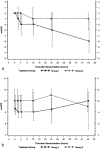
PaO2/FIO2 changes from baseline in the group of patients with an initial PaO2/FIO2 ratio less than and greater than 65 (dotted lines) in the surfactant group (group 1) and controls group (group 2)
Fig. 6.
PaO2/FIO2 changes from baseline in the group of patients with (dotted lines) and without pneumonia in the surfactant group (group 1) and controls (group 2)
Similar articles
- Current definitions of acute lung injury and the acute respiratory distress syndrome do not reflect their true severity and outcome.
Villar J, Pérez-Méndez L, Kacmarek RM. Villar J, et al. Intensive Care Med. 1999 Sep;25(9):930-5. doi: 10.1007/s001340050984. Intensive Care Med. 1999. PMID: 10501747 - Assessment of PaO₂/FiO₂ for stratification of patients with moderate and severe acute respiratory distress syndrome.
Villar J, Blanco J, del Campo R, Andaluz-Ojeda D, Díaz-Domínguez FJ, Muriel A, Córcoles V, Suárez-Sipmann F, Tarancón C, González-Higueras E, López J, Blanch L, Pérez-Méndez L, Fernández RL, Kacmarek RM; Spanish Initiative for Epidemiology, Stratification & Therapies for ARDS (SIESTA) Network. Villar J, et al. BMJ Open. 2015 Mar 27;5(3):e006812. doi: 10.1136/bmjopen-2014-006812. BMJ Open. 2015. PMID: 25818272 Free PMC article. Clinical Trial. - Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis.
Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Pesenti A, Guérin C, Mancebo J, Curley MA, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L. Sud S, et al. Intensive Care Med. 2010 Apr;36(4):585-99. doi: 10.1007/s00134-009-1748-1. Epub 2010 Feb 4. Intensive Care Med. 2010. PMID: 20130832 Review. - Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome.
Stevens TP, Blennow M, Soll RF. Stevens TP, et al. Cochrane Database Syst Rev. 2004;(3):CD003063. doi: 10.1002/14651858.CD003063.pub2. Cochrane Database Syst Rev. 2004. PMID: 15266470 Updated. Review.
Cited by
- Year in review in intensive care medicine-2003. Part 3: intensive care unit organization, scoring, quality of life, ethics, neonatal and pediatrics, and experimental.
Abraham E, Andrews P, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pinsky M, Radermacher P, Ranieri M, Richard C, Tasker R, Vallet B. Abraham E, et al. Intensive Care Med. 2004 Aug;30(8):1514-25. doi: 10.1007/s00134-004-2358-6. Epub 2004 Jun 26. Intensive Care Med. 2004. PMID: 15292983 Review. No abstract available. - Effect of High-Frequency Oscillatory Ventilation Combined With Pulmonary Surfactant in the Treatment of Acute Respiratory Distress Syndrome After Cardiac Surgery: A Prospective Randomised Controlled Trial.
Zheng YR, Lei YQ, Liu JF, Wu HL, Xu N, Huang ST, Cao H, Chen Q. Zheng YR, et al. Front Cardiovasc Med. 2021 Jul 22;8:675213. doi: 10.3389/fcvm.2021.675213. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34368243 Free PMC article. - Persurf, a new method to improve surfactant delivery: a study in surfactant depleted rats.
Burkhardt W, Kraft S, Ochs M, Proquitté H, Mense L, Rüdiger M. Burkhardt W, et al. PLoS One. 2012;7(10):e47923. doi: 10.1371/journal.pone.0047923. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082229 Free PMC article. - Pediatric Acute Respiratory Distress Syndrome: Fibrosis versus Repair.
Im D, Shi W, Driscoll B. Im D, et al. Front Pediatr. 2016 Mar 30;4:28. doi: 10.3389/fped.2016.00028. eCollection 2016. Front Pediatr. 2016. PMID: 27066462 Free PMC article. Review. - Current status of severe acute respiratory syndrome in China.
Nie QH, Luo XD, Zhang JZ, Su Q. Nie QH, et al. World J Gastroenterol. 2003 Aug;9(8):1635-45. doi: 10.3748/wjg.v9.i8.1635. World J Gastroenterol. 2003. PMID: 12918094 Free PMC article.
References
- Bernard Intensive Care Med. 1994;20:225. - PubMed
- Artigas Intensive Care Med. 1998;24:378. - PubMed
- Pfenniger J Pediatr. 1982;101:352. - PubMed
- Kühl Monatsschr Kinderheilkd. 1996;144:1110. doi: 10.1007/s001120050074. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources