Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action - PubMed (original) (raw)
Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action
Hui-Lin Pan et al. J Neurosci. 2003.
Abstract
Resiniferatoxin (RTX), an ultrapotent analog of capsaicin, has been used as a tool to study the role of capsaicin-sensitive C fibers in pain. Recently, we found that RTX diminished the thermal sensitivity but unexpectedly increased the sensitivity to tactile stimulation in adult rats. In this study, we explored the potential mechanisms involved in RTX-induced changes in somatosensory function. An intraperitoneal injection of 200 microg/kg RTX, but not its vehicle, rapidly produced an increase in the paw withdrawal latency to a heat stimulus. Also, profound tactile allodynia developed in all the RTX-treated rats in 3 weeks. This paradoxical change in thermal and mechanical sensitivities lasted for at least 6 weeks. Electron microscopic examination of the sciatic nerve revealed a loss of unmyelinated fibers and extensive ultrastructural damage of myelinated fibers in RTX-treated rats. Immunofluorescence labeling showed a diminished vanilloid receptor 1 immunoreactivity in dorsal root ganglia neurons and the spinal dorsal horn of RTX-treated rats. Furthermore, two transganglionic tracers, horseradish peroxidase conjugates of cholera toxin B subunit (CTB) and isolectin-B(4) of Bandeiraea simplicifolia (IB(4)), were injected into the opposite sides of the sciatic nerve to trace myelinated and unmyelinated afferent terminations, respectively, in the spinal dorsal horn. In RTX-treated rats, IB(4)-labeled terminals in the dorsal horn were significantly reduced, and CTB-labeled terminals appeared to sprout into lamina II of the spinal dorsal horn. Thus, this study demonstrates that systemic RTX diminishes the thermal pain sensitivity by depletion of unmyelinated afferent neurons. The delayed tactile allodynia induced by RTX is likely attributable to damage to myelinated afferent fibers and their abnormal sprouting in lamina II of the spinal dorsal horn. These data provide new insights into the potential mechanisms of postherpetic neuralgia.
Figures
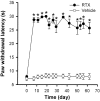
Fig. 1.
Time course of the paw withdrawal latency to a noxious heat stimulus in 6 vehicle- and 10 RTX-treated rats. *p < 0.05 compared with the pretreatment control. The paw withdrawal latency was determined by a radiant heat stimulus.
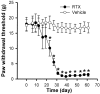
Fig. 2.
Time course of the development of tactile allodynia in 10 rats treated with RTX and the mechanical withdrawal threshold in 6 vehicle-treated rats. *p < 0.05 compared with the pretreatment control. The paw withdrawal thresholds were determined using von Frey filaments.
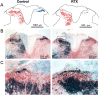
Fig. 3.
Representative electron photomicrographs showing ultrastructural changes of myelinated fibers in the sciatic nerve in a vehicle (A)- and an RTX (B–D)-treated rats. Note that the ultrastructural changes induced by RTX include the loss of unmyelinated fibers and noticeable swelling of the myelinated fibers. Magnification, ×4439.
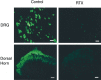
Fig. 4.
VR1 receptor immunoreactivity in lumbar DRG and spinal dorsal horn in a vehicle- and an RTX-treated rat. Densely stained VR1 receptor immunoreactivity is present in the DRG and superficial dorsal horn in the vehicle- but not in the RTX-treated rat. Scale bar, 100 μm.
Fig. 5.
Photomicrographs showing reconstructed plots (A) and the original staining photomicrographs (B) of CTB- and IB4-labeled afferent terminals in the spinal dorsal horn of a vehicle control and an RTX-treated rat. In A and B, the left side of the dorsal horn contains CTB-labeled terminals and the right side contains IB4-labeled afferent terminals.C, Magnification of the inset in_B_. Note that the scattered CTB-labeled terminals are present in lamina II of the RTX-treated rat (C).
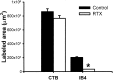
Fig. 6.
Mean amount of CTB- and IB4-labeled areas in the dorsal horn of lumbar spinal sections in two vehicle- (26 sections) and three RTX-treated (40 sections) rats. The mean area was calculated from reconstructed sections as shown in Figure5_A_. Data are presented as means ± SEM. *p < 0.05 compared with the control in the IB4 group (Student's t test).
Similar articles
- Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia.
Wu CH, Lv ZT, Zhao Y, Gao Y, Li JQ, Gao F, Meng XF, Tian B, Shi J, Pan HL, Li M. Wu CH, et al. Mol Pain. 2013 Apr 3;9:18. doi: 10.1186/1744-8069-9-18. Mol Pain. 2013. PMID: 23551937 Free PMC article. - Perineural capsaicin induces the uptake and transganglionic transport of choleratoxin B subunit by nociceptive C-fiber primary afferent neurons.
Oszlács O, Jancsó G, Kis G, Dux M, Sántha P. Oszlács O, et al. Neuroscience. 2015 Dec 17;311:243-52. doi: 10.1016/j.neuroscience.2015.10.042. Epub 2015 Oct 28. Neuroscience. 2015. PMID: 26520849 - Netrin-1 Contributes to Myelinated Afferent Fiber Sprouting and Neuropathic Pain.
Wu CH, Yuan XC, Gao F, Li HP, Cao J, Liu YS, Yu W, Tian B, Meng XF, Shi J, Pan HL, Li M. Wu CH, et al. Mol Neurobiol. 2016 Oct;53(8):5640-51. doi: 10.1007/s12035-015-9482-x. Epub 2015 Oct 19. Mol Neurobiol. 2016. PMID: 26482371 - Injury to dorsal root ganglia alters innervation of spinal cord dorsal horn lamina involved in nociception.
Nakamura SI, Myers RR. Nakamura SI, et al. Spine (Phila Pa 1976). 2000 Mar 1;25(5):537-42. doi: 10.1097/00007632-200003010-00002. Spine (Phila Pa 1976). 2000. PMID: 10749628 - Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity.
Kissin I. Kissin I. Anesth Analg. 2008 Jul;107(1):271-81. doi: 10.1213/ane.0b013e318162cfa3. Anesth Analg. 2008. PMID: 18635498 Free PMC article. Review.
Cited by
- The dual role of TRPV1 in peripheral neuropathic pain: pain switches caused by its sensitization or desensitization.
Gao N, Li M, Wang W, Liu Z, Guo Y. Gao N, et al. Front Mol Neurosci. 2024 Sep 9;17:1400118. doi: 10.3389/fnmol.2024.1400118. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39315294 Free PMC article. Review. - Calcineurin and CK2 Reciprocally Regulate Synaptic AMPA Receptor Phenotypes via α2δ-1 in Spinal Excitatory Neurons.
Huang 黄玉莹 Y, Shao 邵建英 JY, Chen 陈红 H, Zhou 周京京 JJ, Chen 陈少瑞 SR, Pan 潘惠麟 HL. Huang 黄玉莹 Y, et al. J Neurosci. 2024 Jul 17;44(29):e0392242024. doi: 10.1523/JNEUROSCI.0392-24.2024. J Neurosci. 2024. PMID: 38886057 - Analysis of the Risk Factors for Mechanical Allodynia in Herpetic Neuralgia: A Retrospective Cross-Sectional Study.
Xu G, Gong W, Dong S, Hu G, Tang W, Yu H. Xu G, et al. J Pain Res. 2023 Oct 2;16:3309-3318. doi: 10.2147/JPR.S417454. eCollection 2023. J Pain Res. 2023. PMID: 37808462 Free PMC article. - Brief Opioid Exposure Paradoxically Augments Primary Afferent Input to Spinal Excitatory Neurons via α2δ-1-Dependent Presynaptic NMDA Receptors.
Chen SR, Chen H, Jin D, Pan HL. Chen SR, et al. J Neurosci. 2022 Dec 14;42(50):9315-9329. doi: 10.1523/JNEUROSCI.1704-22.2022. Epub 2022 Nov 15. J Neurosci. 2022. PMID: 36379705 Free PMC article. - Elucidating the Ability of CGRP to Modulate Microvascular Events in Mouse Skin.
Zarban AA, Chaudhry H, de Sousa Valente J, Argunhan F, Ghanim H, Brain SD. Zarban AA, et al. Int J Mol Sci. 2022 Oct 13;23(20):12246. doi: 10.3390/ijms232012246. Int J Mol Sci. 2022. PMID: 36293102 Free PMC article.
References
- Alloway KD, Mutic JJ, Hoffer ZS, Hoover JE. Overlapping corticostriatal projections from the rodent vibrissal representations in primary and secondary somatosensory cortex. J Comp Neurol. 2000;428:51–67. - PubMed
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. - PubMed
- Bao L, Wang HF, Cai HJ, Tong YG, Jin SX, Lu YJ, Grant G, Hokfelt T, Zhang X. Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur J Neurosci. 2002;16:175–185. - PubMed
- Baron R, Saguer M. Postherpetic neuralgia: are C-nociceptors involved in signaling and maintenance of tactile allodynia? Brain. 1993;116:1477–1496. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM64830/GM/NIGMS NIH HHS/United States
- HL04199/HL/NHLBI NIH HHS/United States
- K02 HL004199/HL/NHLBI NIH HHS/United States
- NS41178/NS/NINDS NIH HHS/United States
- R01 GM064830/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources