Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein - PubMed (original) (raw)
Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein
Manuel Yepes et al. J Clin Invest. 2003 Nov.
Abstract
The regulation of cerebrovascular permeability is critical for normal brain homeostasis, and the "breakdown" of the blood-brain barrier (BBB) is associated with the development of vasogenic edema and intracranial hypertension in a number of neurological disorders. In this study we demonstrate that an increase in endogenous tissue-type plasminogen activator (tPA) activity in the perivascular tissue following cerebral ischemia induces opening of the BBB via a mechanism that is independent of both plasminogen (Plg) and MMP-9. We also show that injection of tPA into the cerebrospinal fluid in the absence of ischemia results in a rapid dose-dependent increase in vascular permeability. This activity is not seen with urokinase-type Plg activator (uPA) but is induced in Plg-/- mice, confirming that the effect is Plg-independent. However, the activity is blocked by antibodies to the LDL receptor-related protein (LRP) and by the LRP antagonist, receptor-associated protein (RAP), suggesting a receptor-mediated process. Together these studies demonstrate that tPA is both necessary and sufficient to directly increase vascular permeability in the early stages of BBB opening, and suggest that this occurs through a receptor-mediated cell signaling event and not through generalized degradation of the vascular basement membrane.
Figures
Figure 1
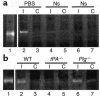
MMP-9 activity in brain extracts following cerebral ischemia. (a) Zymographic assay of brain extracts from rats following MCAO. Lane 1 is purified human proMMP-9 and all other lanes show MMP-9 activity 6 hours after MCAO in rats that were intracortically injected ipsilateral to the ischemic area immediately following MCAO with either PBS (lanes 2 and 3) or neuroserpin (Ns, lanes 4–7). Lanes 2, 4, and 6 are ipsilateral (I) to the ischemic area and lanes 3, 5, and 7 are contralateral (C). (b) Zymographic assay of brain extracts from mice following MCAO. Lane 1 is purified human proMMP-9. The other lanes correspond to brain extracts from WT C57BL/6J (lane 2 and lane 3), tPA–/– C57BL/6J (lane 4 and lane 5), and Plg –/– C57BL/6J (lane 6 and lane 7) mice 6 hours after MCAO. Lanes 2, 4, and 6 are ipsilateral to the ischemic area and lanes 3, 5, and 7 are contralateral.
Figure 2
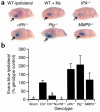
BBB permeability following cerebral ischemia. (a) Analysis of Evans blue dye extravasation in mice. Brains were removed and photographed 6 hours after MCAO. All images are ipsilateral to the ischemic area. WT ipsilateral is a WT C57BL/6J mouse, and WT + Ns is a WT C57BL/6J mouse treated with an intraventricular injection of 2.5 μl of 16 μM neuroserpin immediately after MCAO. _tPA_–/–, _uPA_–/–, _Plg_–/–, and _MMP9_–/– are mice deficient in the enzyme indicated. The arrows indicate the area of Evans blue extravasation associated with the ischemic injury. (b) Quantitative analysis of Evans blue extravasation from brain extracts 6 hours after MCAO. Sham indicates animals subjected to all procedures except MCA ligation. The results represent the specific absorbance of Evans blue at 620 nm calculated as percentage of that of the WT control, either C57BL/6J (C57) or 129S6/SvEv as described in Methods. As a control for perfusion efficiency, the absorbance of the contralateral hemisphere was subtracted from that of the hemisphere ipsilateral to the MCAO. For each condition, n = 4. *P < 0.05 relative to WT control mice receiving MCAO.
Figure 3
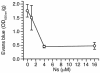
Dose-response study of neuroserpin treatment following MCAO. Quantitative analysis of Evans blue extravasation from brain extracts 6 hours after MCAO in WT C57BL/6J mice treated with an intraventricular injection of 2.5 μl of either PBS or increasing doses of neuroserpin immediately after MCAO. Analysis was as in Figure 2, and n = 4–6 for each point.
Figure 4
Temporal and spatial relationship between tPA activity and vascular permeability following MCAO in WT (C57BL/6J) mice. In a–d, tPA activity 1 hour after MCAO is shown in red by in situ zymography, and cell nuclei are in blue (DAPI). (a) By 1 hour after MCAO there is significant tPA activity in the vessel wall and in the perivascular tissue surrounding a vessel bordering the necrotic area. (b and c) The same vessel in adjacent sections (5 μm), but with anti-tPA antibodies included (b), or without the addition of Plg (c) in the overlay. (d) The background tPA activity associated with a vessel in a corresponding area in the contralateral hemisphere from the same section shown in a. In a–d the original magnification was ×100. (e–h) Evans blue extravasation is shown in red and cell nuclei in blue (DAPI) 6 hours after MCAO. (e) A low-magnification view of the entire ischemic area. (f) Evans blue extravasation from a vessel located in the area adjacent to the ischemic area, similar to the one seen in a. (g) Electronic magnification of the box in f. The arrow indicates an area of Evans blue leakage outside the internal elastic lamina of the vessel. (h) Evans blue is shown adhering to the vessel wall, but no extravasation is seen in a vessel from the same section seen in f and g but located in the corresponding region of the contralateral hemisphere. Ipsi, ipsilateral; Contra, contralateral.
Figure 5
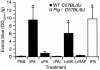
Changes in vascular permeability following intraventricular injection of tPA, determined by quantitative analysis of Evans blue extravasation from brain extracts. Measurements were performed 1 hour after intraventricular injection of 2.5 μl of either PBS, active tPA (tPA, 0.7 μM), inactive tPA (tPAi, 0.7 μM), active uPA (uPA, 0.9 μM), and active tPA following 1 hour after pretreatment with the NMDA receptor antagonist MK-801 (t+MK) or coinjection of 5 μl of 0.35 μM active tPA in combination with a 25-fold molar excess (9 μM) of the LRP antagonist RAP (t+RAP). The black bars indicate WT C57BL/6J mice and the white bar indicates _Plg_–/– C57BL/6J mice. For each condition, n = 6–8. *P < 0.01 vs. animals injected with PBS.
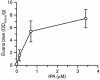
Figure 6
Analysis of Evans blue extravasation following a dose-response study of intraventricular injection of tPA. Quantitative analysis of Evans blue extravasation from brain extracts of WT C57BL/6J mice 1 hour after intraventricular injection of 2.5 μl of increasing concentrations of active tPA. n = 6 for each point.
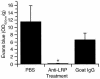
Figure 7
Effect of anti-LRP antibodies on tPA-induced cerebrovascular permeability determined by quantitative analysis of Evans blue extravasation from brain extracts of WT C57BL/6J mice 1 hour after intraventricular coinjection of 5 μl of 0.35 μM tPA with either PBS, purified goat anti-LRP IgG (85 μg/ml), or normal goat IgG (85 μg/ml). n = 6 for each point. *P < 0.01 vs. animals injected with PBS.
Comment in
- LRP: a bright beacon at the blood-brain barrier.
Herz J. Herz J. J Clin Invest. 2003 Nov;112(10):1483-5. doi: 10.1172/JCI20337. J Clin Invest. 2003. PMID: 14617749 Free PMC article.
Similar articles
- Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse.
Copin JC, Bengualid DJ, Da Silva RF, Kargiotis O, Schaller K, Gasche Y. Copin JC, et al. Eur J Neurosci. 2011 Oct;34(7):1085-92. doi: 10.1111/j.1460-9568.2011.07843.x. Epub 2011 Sep 6. Eur J Neurosci. 2011. PMID: 21895804 - Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process.
Benchenane K, Berezowski V, Fernández-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C. Benchenane K, et al. Stroke. 2005 May;36(5):1065-70. doi: 10.1161/01.STR.0000163050.39122.4f. Epub 2005 Apr 7. Stroke. 2005. PMID: 15817895 - Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain.
Zhang C, An J, Haile WB, Echeverry R, Strickland DK, Yepes M. Zhang C, et al. J Cereb Blood Flow Metab. 2009 Dec;29(12):1946-54. doi: 10.1038/jcbfm.2009.174. Epub 2009 Aug 12. J Cereb Blood Flow Metab. 2009. PMID: 19672275 - tPA in the central nervous system: relations between tPA and cell surface LRPs.
Ortolano S, Spuch C. Ortolano S, et al. Recent Pat Endocr Metab Immune Drug Discov. 2013 Jan;7(1):65-76. Recent Pat Endocr Metab Immune Drug Discov. 2013. PMID: 23231415 Review. - New functions for an old enzyme: nonhemostatic roles for tissue-type plasminogen activator in the central nervous system.
Yepes M, Lawrence DA. Yepes M, et al. Exp Biol Med (Maywood). 2004 Dec;229(11):1097-104. doi: 10.1177/153537020422901103. Exp Biol Med (Maywood). 2004. PMID: 15564435 Review.
Cited by
- Tissue-Type Plasminogen Activator-A296-299 Prevents Impairment of Cerebral Autoregulation After Stroke Through Lipoprotein-Related Receptor-Dependent Increase in cAMP and p38.
Armstead WM, Riley J, Yarovoi S, Higazi AA, Cines DB. Armstead WM, et al. Stroke. 2016 Aug;47(8):2096-102. doi: 10.1161/STROKEAHA.116.012678. Epub 2016 Jun 28. Stroke. 2016. PMID: 27354223 Free PMC article. - Tissue Plasminogen Activator: Side Effects and Signaling.
Lin L, Hu K. Lin L, et al. J Drug Des Res. 2014 Sep 25;1(1):1001. J Drug Des Res. 2014. PMID: 25879083 Free PMC article. No abstract available. - Passenger mutations and aberrant gene expression in congenic tissue plasminogen activator-deficient mouse strains.
Szabo R, Samson AL, Lawrence DA, Medcalf RL, Bugge TH. Szabo R, et al. J Thromb Haemost. 2016 Aug;14(8):1618-28. doi: 10.1111/jth.13338. Epub 2016 Jun 20. J Thromb Haemost. 2016. PMID: 27079292 Free PMC article. - LDL receptor-related protein 1 regulates the abundance of diverse cell-signaling proteins in the plasma membrane proteome.
Gaultier A, Simon G, Niessen S, Dix M, Takimoto S, Cravatt BF 3rd, Gonias SL. Gaultier A, et al. J Proteome Res. 2010 Dec 3;9(12):6689-95. doi: 10.1021/pr1008288. Epub 2010 Oct 28. J Proteome Res. 2010. PMID: 20919742 Free PMC article. - Modeling multiple sclerosis in laboratory animals.
Schreiner B, Heppner FL, Becher B. Schreiner B, et al. Semin Immunopathol. 2009 Nov;31(4):479-95. doi: 10.1007/s00281-009-0181-4. Epub 2009 Oct 3. Semin Immunopathol. 2009. PMID: 19802608 Review.
References
- Bugge TH, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. - PubMed
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. - PubMed
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-02223/NS/NINDS NIH HHS/United States
- K08 NS002223/NS/NINDS NIH HHS/United States
- P01 HL054710/HL/NHLBI NIH HHS/United States
- HL-54710/HL/NHLBI NIH HHS/United States
- HL-55747/HL/NHLBI NIH HHS/United States
- R01 HL055747/HL/NHLBI NIH HHS/United States
- HL-55374/HL/NHLBI NIH HHS/United States
- R01 HL055374/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous