Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance - PubMed (original) (raw)
Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance
S L Singla-Pareek et al. Proc Natl Acad Sci U S A. 2003.
Erratum in
- Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6326
Abstract
The glyoxalase pathway involving glyoxalase I (gly I) and glyoxalase II (gly II) enzymes is required for glutathione-based detoxification of methylglyoxal. We had earlier indicated the potential of gly I as a probable candidate gene in conferring salinity tolerance. We report here that overexpression of gly I+II together confers improved salinity tolerance, thus offering another effective strategy for manipulating stress tolerance in crop plants. We have overexpressed the gly II gene either alone in untransformed plants or with gly I transgenic background. Both types of these transgenic plants stably expressed the foreign protein, and the enzyme activity was also higher. Compared with nontransformants, several independent gly II transgenic lines showed improved capability for tolerating exposure to high methylglyoxal and NaCl concentration and were able to grow, flower, and set normal viable seeds under continuous salinity stress conditions. Importantly, the double transgenic lines always showed a better response than either of the single gene-transformed lines and WT plants under salinity stress. Ionic measurements revealed higher accumulation of Na+ and K+ in old leaves and negligible accumulation of Na+ in seeds of transgenic lines as compared with the WT plants. Comparison of various growth parameters and seed production demonstrated that there is hardly any yield penalty in the double transgenics under nonstress conditions and that these plants suffered only 5% loss in total productivity when grown in 200 mM NaCl. These findings establish the potential of manipulation of the glyoxalase pathway for increased salinity tolerance without affecting yield in crop plants.
Figures
Fig. 1.
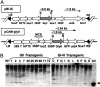
Transformation of tobacco by using glyoxalase pathway genes. (A) Schematic representation of various glyoxalase constructs used to overexpress gly I enzyme (pBI-SI) and gly II enzyme (pCAM-glyII) in tobacco plants. (B) Testing of various gly II (GII) and double (GI+II) transgenic lines for the presence of gly II transgene by Southern hybridization. The line number of each type of transgenic is given at the top. The presence of a 1.0-kb band on the Southern blot is shown by an arrowhead.
Fig. 2.
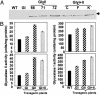
(A) Western blot analysis of WT and various glyoxalase transgenic plants (GI, gly I; GlyII, gly II; and GlyI+II, double transgenics) carried out with antibodies raised against rice gly II protein. The line number of each type of transgenic is given at the top. The presence of 36-kDa gly II protein is marked with an arrow. (B) Histograms showing the activity of the gly II (Left) and gly I (Right) enzymes in the selected transgenic lines: NtBIS-11 for gly I; line 66 (Upper Left) and 72 (Lower Left) for gly II; and line C (Upper Right) and K (Lower Right) for glyI+II. The standard deviation is indicated by each bar in the graph (n = 3).
Fig. 3.
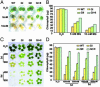
Retardation of MG- and salt stress-promoted senescence in transgenic tobacco plants overexpressing either gly I (GI), gly II (GII), or both gly I and II in double transgenics (GI+II), indicating the tolerance at cellular levels toward toxic levels of MG and salt. Phenotypic differences (A) and chlorophyll content (B) (μg/g of fresh weight) from MG-treated leaf discs of WT and various transgenic plants (GI, GII, and GI+II) after incubation in 5 and 10 mM solutions of MG for 48 h are shown. Discs floated in water served as the experimental control. Phenotypic differences (C) and chlorophyll content (D)(μg/g of fresh weight) from sodium chloride-treated leaf discs of WT and various transgenic plants (GI, GII, and GI+II) after incubation in 400 and 800 mM solutions of NaCl for 3 and 5 days are shown. Discs floated in water served as the experimental control. The standard deviation in each case is represented by the vertical bar in each graph (n = 3). Note the difference in retention of chlorophyll in WT and transgenic plants.
Fig. 4.
Relative salt tolerance of WT and glyoxalase-overexpressing transgenic T1 generation tobacco plants (GI, gly I; GII, gly II; and GI+II, double transgenics) at seedling and whole mature plant level. (A) Seedlings were grown on medium supplemented with 0, 100, 200, and 400 mM NaCl for 25 days. (B) WT and transgenic plants were grown in the continued presence of 200 mM NaCl for 98 days. Note that WT plants could not sustain growth under this condition.
Fig. 5.
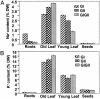
Na+ (A) and K+ (B) content [calculated as percent dry weight (DW) of the tissue] in various tissues of the glyoxalase transgenic plants (GI, gly I; GII, gly II; and GIGII, double transgenics) grown under the continued presence of 200 mM NaCl. In the histogram, each of the transgenic types is indicated by different patterned bars as shown. For each determination, roots, old leaf (fourth leaf from the bottom), young leaf (second leaf from the top), and seeds were collected from three different plants of each type. Values are the mean ± standard deviation (n = 3). Similar data for WT plants could not be obtained as these plants did not grow further in the presence of 200 mM NaCl. However, the relative values for Na+ in the WT plants grown in water were found to be 0.5% in roots, 0.1% in old leaf, 0.4% in young leaf, and 0.05% in seeds, and those for K+ were 1.5% in roots, 1.8% in old leaf, 2.0% in young leaf, and 1.0% in seeds (data not shown).
Similar articles
- Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils.
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Singla-Pareek SL, et al. Plant Physiol. 2006 Feb;140(2):613-23. doi: 10.1104/pp.105.073734. Epub 2005 Dec 29. Plant Physiol. 2006. PMID: 16384901 Free PMC article. - Overexpression of a glyoxalase gene, OsGly I, improves abiotic stress tolerance and grain yield in rice (Oryza sativa L.).
Zeng Z, Xiong F, Yu X, Gong X, Luo J, Jiang Y, Kuang H, Gao B, Niu X, Liu Y. Zeng Z, et al. Plant Physiol Biochem. 2016 Dec;109:62-71. doi: 10.1016/j.plaphy.2016.09.006. Epub 2016 Sep 13. Plant Physiol Biochem. 2016. PMID: 27639962 - Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress.
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK. Yadav SK, et al. FEBS Lett. 2005 Nov 7;579(27):6265-71. doi: 10.1016/j.febslet.2005.10.006. Epub 2005 Oct 17. FEBS Lett. 2005. PMID: 16253241 - Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants.
Hasanuzzaman M, Nahar K, Hossain MS, Mahmud JA, Rahman A, Inafuku M, Oku H, Fujita M. Hasanuzzaman M, et al. Int J Mol Sci. 2017 Jan 20;18(1):200. doi: 10.3390/ijms18010200. Int J Mol Sci. 2017. PMID: 28117669 Free PMC article. Review. - An overview on the role of methylglyoxal and glyoxalases in plants.
Yadav SK, Singla-Pareek SL, Sopory SK. Yadav SK, et al. Drug Metabol Drug Interact. 2008;23(1-2):51-68. doi: 10.1515/dmdi.2008.23.1-2.51. Drug Metabol Drug Interact. 2008. PMID: 18533364 Review.
Cited by
- Large-scale proteome comparative analysis of developing rhizomes of the ancient vascular plant equisetum hyemale.
Balbuena TS, He R, Salvato F, Gang DR, Thelen JJ. Balbuena TS, et al. Front Plant Sci. 2012 Jun 26;3:131. doi: 10.3389/fpls.2012.00131. eCollection 2012. Front Plant Sci. 2012. PMID: 22740841 Free PMC article. - A DESD-box helicase functions in salinity stress tolerance by improving photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. PB1).
Gill SS, Tajrishi M, Madan M, Tuteja N. Gill SS, et al. Plant Mol Biol. 2013 May;82(1-2):1-22. doi: 10.1007/s11103-013-0031-6. Epub 2013 Feb 28. Plant Mol Biol. 2013. PMID: 23456247 - Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance.
Pinedo I, Ledger T, Greve M, Poupin MJ. Pinedo I, et al. Front Plant Sci. 2015 Jun 23;6:466. doi: 10.3389/fpls.2015.00466. eCollection 2015. Front Plant Sci. 2015. PMID: 26157451 Free PMC article. - Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress.
Alvarez Viveros MF, Inostroza-Blancheteau C, Timmermann T, González M, Arce-Johnson P. Alvarez Viveros MF, et al. Mol Biol Rep. 2013 Apr;40(4):3281-90. doi: 10.1007/s11033-012-2403-4. Epub 2013 Jan 3. Mol Biol Rep. 2013. PMID: 23283739 - Transgene Stacking as Effective Tool for Enhanced Disease Resistance in Plants.
Shehryar K, Khan RS, Iqbal A, Hussain SA, Imdad S, Bibi A, Hamayun L, Nakamura I. Shehryar K, et al. Mol Biotechnol. 2020 Jan;62(1):1-7. doi: 10.1007/s12033-019-00213-2. Mol Biotechnol. 2020. PMID: 31538309 Review.
References
- Altman, A. (1999) Electronic J. Biotechnol. 2, 51–55.
- Cushman, J. C. & Bohnert, H. J. (2000) Curr. Opin. Plant Biol. 3, 117–124. - PubMed
- Hasegawa, P. M., Bressan, R. A., Zhu, J. K. & Bohnert, H. J. (2000) Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 463–499. - PubMed
- Singla-Pareek, S. L., Reddy, M. K. & Sopory, S. K. (2001) Proc. Indian Natl. Sci. Acad. 67, 265–284.
- Datta, S. K. (2002) in Genetic Engineering of Crop Plants for Abiotic Stress, JIRCAS Working Report, ed. Iwanaga, M. (Japan International Research Center for Agricultural Sciences, Ibaraki), pp. 43–53.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources