Epithelial-mesenchymal transition and its implications for fibrosis - PubMed (original) (raw)
Review
Epithelial-mesenchymal transition and its implications for fibrosis
Raghu Kalluri et al. J Clin Invest. 2003 Dec.
Abstract
Epithelial to mesenchymal transition (EMT) is a central mechanism for diversifying the cells found in complex tissues. This dynamic process helps organize the formation of the body plan, and while EMT is well studied in the context of embryonic development, it also plays a role in the genesis of fibroblasts during organ fibrosis in adult tissues. Emerging evidence from studies of renal fibrosis suggests that more than a third of all disease-related fibroblasts originate from tubular epithelia at the site of injury. This review highlights recent advances in the process of EMT signaling in health and disease and how it may be attenuated or reversed by selective cytokines and growth factors.
Figures
Figure 1
Primitive epithelia (epiblasts) form tropoblastic germ layers through EMT. The primary mesenchyme that migrates after EMT is reinduced to secondary epithelium by mesenchymal-epithelial transition. Secondary epithelia differentiate to form new epithelial tissues and undergo a second round of EMT to form the cells of connective tissue, including astrocytes, adipocytes chondrocytes, osteoblasts, muscle cells, and fibroblasts. Mature secondary epithelia that form epithelial organs can also transform into primary tumors that later undergo EMT to metastasize. These processes are regulated by morphogenic cues and a variety of transcription factors, and are potentially plastic in their adaptation to new biologic circumstances.
Figure 2
Epithelial plasticity can lead to classical EMT (loss of cell-cell and cell-substratum attachments, new actin rearrangements, and gain of mobility) or reversible scatter, which looks like EMT but is not enduring and can revert. These events are regulated by ligand-inducible intrinsic kinase receptors on the cell surface, which modulate small GTPases, Smads, PI3Ks, MAP kinases, and the availability of β-catenin to coactivate LEF in the nucleus. Free levels of b-catenin are regulated by E-cadherin or APC/β-catenin/Axin complexes, the latter of which shuttle b-catenin between ubiquination or utilization in adherens junctions. Activation of nuclear transcription provides new transcriptional regulators (Snail, SIP1, Ets, and FTS-BP/CarG box binding factor) of the EMT proteome. The EMT proteome comprises proteins listed in Table 1. The variability of receptors, kinases, and the emergence of combined preferences for signaling pathways determine the plasticity unique to each epithelium.
Figure 3
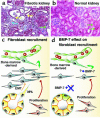
Origin of fibroblasts during kidney fibrosis. (a) Fibrotic kidney which displays accumulation of numerous fibroblasts (blue arrow), damaged kidney tubules (yellow arrow), and blood vessels (green arrow). (b) Normal kidney with proper tubular structures and very few fibroblasts. (c) Schematic illustration of three possible mechanisms via which fibroblasts can originate during kidney injury. Recent experiments suggest that approximately 14–15% of fibroblasts are from bone marrow, 36% can arise via local EMT involving tubular epithelial cells under inflammatory stress, and the rest are likely contributed by proliferation of fibroblasts from all sources. (d) Systemic treatment of mice with renal fibrosis using recombinant human BMP-7 results in reversal of renal disease due to severe decrease in EMT-derived fibroblasts and potentially bone marrow–derived fibroblasts. Such events likely have a cascade of beneficial effects that decreasing the overall number of fibroblasts in the kidney, and attenuating fibrosis.
Similar articles
- Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy.
Bani-Hani AH, Campbell MT, Meldrum DR, Meldrum KK. Bani-Hani AH, et al. J Urol. 2008 Aug;180(2):461-8. doi: 10.1016/j.juro.2008.04.001. Epub 2008 Jun 11. J Urol. 2008. PMID: 18550128 Review. - The role of EMT in renal fibrosis.
Carew RM, Wang B, Kantharidis P. Carew RM, et al. Cell Tissue Res. 2012 Jan;347(1):103-16. doi: 10.1007/s00441-011-1227-1. Epub 2011 Aug 16. Cell Tissue Res. 2012. PMID: 21845400 Review. - Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis.
Zeisberg M, Kalluri R. Zeisberg M, et al. Front Biosci. 2008 May 1;13:6991-8. doi: 10.2741/3204. Front Biosci. 2008. PMID: 18508710 - Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention.
Liu Y. Liu Y. J Am Soc Nephrol. 2004 Jan;15(1):1-12. doi: 10.1097/01.asn.0000106015.29070.e7. J Am Soc Nephrol. 2004. PMID: 14694152 Review. - Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition.
Guarino M, Tosoni A, Nebuloni M. Guarino M, et al. Hum Pathol. 2009 Oct;40(10):1365-76. doi: 10.1016/j.humpath.2009.02.020. Epub 2009 Aug 19. Hum Pathol. 2009. PMID: 19695676 Review.
Cited by
- Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age.
Roeder SS, Stefanska A, Eng DG, Kaverina N, Sunseri MW, McNicholas BA, Rabinovitch P, Engel FB, Daniel C, Amann K, Lichtnekert J, Pippin JW, Shankland SJ. Roeder SS, et al. Am J Physiol Renal Physiol. 2015 Jul 15;309(2):F164-78. doi: 10.1152/ajprenal.00144.2015. Epub 2015 May 27. Am J Physiol Renal Physiol. 2015. PMID: 26017974 Free PMC article. - TOP2A Promotes Cell Migration, Invasion and Epithelial-Mesenchymal Transition in Cervical Cancer via Activating the PI3K/AKT Signaling.
Wang B, Shen Y, Zou Y, Qi Z, Huang G, Xia S, Gao R, Li F, Huang Z. Wang B, et al. Cancer Manag Res. 2020 May 21;12:3807-3814. doi: 10.2147/CMAR.S240577. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547216 Free PMC article. - The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment.
Ni Y, Zhou X, Yang J, Shi H, Li H, Zhao X, Ma X. Ni Y, et al. Front Cell Dev Biol. 2021 May 20;9:637675. doi: 10.3389/fcell.2021.637675. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095111 Free PMC article. Review. - The Dark Side of Cell Fusion.
Bastida-Ruiz D, Van Hoesen K, Cohen M. Bastida-Ruiz D, et al. Int J Mol Sci. 2016 Apr 28;17(5):638. doi: 10.3390/ijms17050638. Int J Mol Sci. 2016. PMID: 27136533 Free PMC article. Review. - Phf14, a novel regulator of mesenchyme growth via platelet-derived growth factor (PDGF) receptor-α.
Kitagawa M, Takebe A, Ono Y, Imai T, Nakao K, Nishikawa S, Era T. Kitagawa M, et al. J Biol Chem. 2012 Aug 10;287(33):27983-96. doi: 10.1074/jbc.M112.350074. Epub 2012 Jun 23. J Biol Chem. 2012. PMID: 22730381 Free PMC article.
References
- Virchow, R. 1858. Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. A. Hirschwald. Berlin, Germany. 456 pp.
- Slack JMW. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. - PubMed
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. - PubMed
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
- Irvine KD, Rauskolb C. Boundaries in development: formation and function. Annu. Rev. Cell Dev. Biol. 2001;17:189–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK55001/DK/NIDDK NIH HHS/United States
- DK46282/DK/NIDDK NIH HHS/United States
- R01 DK062987/DK/NIDDK NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
- R01 DK046282/DK/NIDDK NIH HHS/United States
- DK62987/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases