Automatic and quantitative measurement of protein-protein colocalization in live cells - PubMed (original) (raw)
Automatic and quantitative measurement of protein-protein colocalization in live cells
Sylvain V Costes et al. Biophys J. 2004 Jun.
Abstract
We introduce a novel statistical approach that quantifies, for the first time, the amount of colocalization of two fluorescent-labeled proteins in an image automatically, removing the bias of visual interpretation. This is done by estimating simultaneously the maximum threshold of intensity for each color below which pixels do not show any statistical correlation. The sensitivity of the method was illustrated on simulated data by statistically confirming the existence of true colocalization in images with as little as 3% colocalization. This method was then tested on a large three-dimensional set of fixed cells cotransfected with CFP/YFP pairs of proteins that either co-compartmentalized, interacted, or were just randomly localized in the nucleolus. In this test, the algorithm successfully distinguished random color overlap from colocalization due to either co-compartmentalization or interaction, and results were verified by fluorescence resonance energy transfer. The accuracy and consistency of our algorithm was further illustrated by measuring, for the first time in live cells, the dissociation rate (k(d)) of the HIV-1 Rev/CRM1 export complex induced by the cytotoxin leptomycin B. Rev/CRM1 colocalization in nucleoli dropped exponentially after addition of leptomycin B at a rate of 1.25 x 10(-3) s(-1). More generally, this algorithm can be used to answer a variety of biological questions involving protein-protein interactions or co-compartmentalization and can be generalized to colocalization of more than two colors.
Figures
FIGURE 1
The automatic threshold search is done in a two-dimensional histogram (shown on left graph) along a line whose slope and intercept (a and b) are obtained by linear least-square fit of the red and green intensities (_I_R and _I_G) over all pixels in the image (i.e., _I_G = a × _I_R + b). The threshold (T) corresponds to two intensity values (T and a × T + b) applied simultaneously to the red and green channels, respectively. Any pixel with red intensities >T and green intensity >a × T + b is said to be above the threshold. Starting with the highest intensity value, the algorithm reduces the threshold value incrementally and computes the correlation coefficient of the image using only pixels with intensities below the threshold. The algorithm continues reducing the threshold until r reaches 0. The corresponding three-dimensional color image for this simulation is shown on the right of the graph (noted No threshold) and the pixels above different threshold are shown as white surfaces. In this simulation, r is 0.4 for threshold _T_1, 0.25 for lower threshold _T_2, and 0 for threshold _T_3. Thus, _T_3 is the automatic threshold our algorithm will return for the red channel and a × _T_3 + b for the green channel. As further discussed in the main text, some pixels are still colocalized (i.e., ellipse shown in red) and some pixels are anti-colocalized (i.e., one channel dim, the other one bright, shown as rectangles) in the r = 0 region (light pink area). However, trying to include the dim colocalized pixels is a difficult task since, as they become dimmer, they are most likely background noise (i.e., shown in dotted circle).
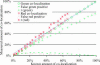
FIGURE 2
In this simulation pixel-sized objects were generated with random positions and colocalized pixels had identical nonzero intensities in both colors. The intensity distribution of all pixels was uniform with a range 100–255 in both channels. A test series of 100 three-dimensional images (30 × 30 × 30) was generated with different fractions of colocalization by controlling the percentage of identical objects in both channels. The choice of the density of each object was set deliberately low and different (i.e., 6% of the area is covered by green objects and 9% by red) to force the amount of red and green colocalization to be different. Simulations covering the full range of possible colocalization were performed, from all green objects colocalized (i.e., 100% and 67% colocalized green and red objects, respectively) to no colocalization. Finally, noise with intensity distributed uniformly between 10 and 30 was added to both channels. The Pearson's correlation coefficient, r, is plotted twice for the same image, once against the real amount of green colocalization (green +) and once against the red amount of colocalization (red *). We can see that r (* or +) evaluates inaccurately either amount of colocalization in comparison to our current algorithm (○ or □). The _P_-value (not shown) remained above the 95% significance level as long as >1% of the green objects were colocalized. For all test images, measured amounts of colocalization were accurate with <2% deviation, as is observed with most points close to the diagonal of slope 1 (dashed line). Finally, the two dashed curves show the fractions of pixels without colocalization that were incorrectly classified as being colocalized. This was worst for intermediate amount of colocalization, where up to 5% of pixels were misclassified.
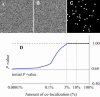
FIGURE 3
Detection of nonrandom colocalization that is not visible. A and B are two independent computer-generated 256 × 256 images with pixel intensities randomly distributed between 0 and 255 except for 3% of the pixels (shown in image C) having the same intensity for both channels. Even though one cannot see which pixels are identical between A and B, our algorithm could identify this difference unequivocally with a true colocalization significance test above the significant threshold of 0.95. D shows the sensitivity of the algorithm as a function of the proportion of colocalization by repeating the process described above for different colocalized images going from 0% to 100% colocalization. This graph shows _P_-values >95% for colocalized amounts as low as 3%. If there is <3% of the image being colocalized, the algorithm returns an inconclusive answer about the existence of colocalization; i.e., _P_-value is <95%. Note on D: if no colocalization was deliberately inserted, i.e., image C was blank, the _P_-value of the two initial uncorrelated images used for this simulation was 0.68. This value has no meaning in itself and could be anywhere between 0 and 1 for two uncorrelated images, with a most probable value ∼0.5 (see Fig. 1s of Supplementary Material for distribution of r).
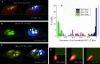
FIGURE 4
Colocalization control study. A_–_C show representative confocal slices of the analyzed HeLa cells (CFP shown in green and YFP in red). On the left of these panels, one center slice is shown and on the right a reconstructed surface of the nuclear membrane is shown in blue. The nucleoli where colocalization was computed are shown as white surfaces. Each group analysis was performed on a population of 40 cells. Group A focused on colocalization of free YFP and Rev-CFP in the nucleoli and had very little colocalization, as shown by the green distribution in D with an average colocalization of 5% for both signals and a _P_-value of 0.7 indicating the random aspect of that colocalization. Group B focused on colocalization of Tat-YFP and Rev-CFP, two proteins known to bind to rRNA. This co-compartmentalization led to an average colocalization of 60% for both signals with a _P_-value >0.995. Finally, group C focused on colocalization of Rev-YFP and Rev-CFP, a protein known to multimerize in addition to binding to rRNA. This combination of co-compartmentalization and interaction led to a very narrow distribution of colocalization in the nucleoli ∼98% for both signals with a _P_-value >0.95. E shows a representative two-dimensional histogram of those different groups within the nucleoli. The red channel (Rev, Tat, or free YFP) is on the x axis and the green (Rev) is on the y axis in the two-dimensional histograms. Automatic selected colocalized areas are shown by a rectangular yellow overlay in the two-dimensional histogram. One can appreciate, in the Rev-Tat case, how difficult it would be to decide manually what threshold levels to use.
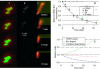
FIGURE 5
Rev-CRM1 dissociation. (A) Time series of two-dimensional confocal sections of a cell cotransfected with CRM1 and Rev fused to YFP (red) and CFP (green), respectively. (B) The corresponding estimate of colocalized pixels (shown in white). (C) The corresponding two-dimensional histograms for each image in A. The red channel (CRM1) is on the x axis and the green one (Rev) on the y axis in the two-dimensional histogram. Selected colocalized areas are also shown by yellow transparent rectangular areas. Initially, CRM1 colocalized with Rev in the nucleolus. Thirty-three minutes after addition of leptomycin B, an inhibitor of the CRM1-cargo binding, most of the CRM1 has dispersed in the nucleus. The last image of the color sequence indicates the region of the nucleolus in which the amount of colocalization is computed (bold dashed line in A). The nucleus and cell limits are also shown (solid and light dashed lines, respectively, in A). (D) The graph shows the relative concentration of the bound complex Rev/CRM1 derived from the amount of colocalization of each protein (expressions in Eq. 12) as a function of time after the end of the lag period. The dissociation rate constant _k_d was found to be (1.25 ± 0.31) × 10−3 s−1 (fit shown by solid line). Error bars are standard errors based on all cells averaged for each time point. Average _P_-values for colocalization significance test are also plotted as ▴ and confirm Rev/CRM1 interaction for at least the first 20 min of dissociation (at a 95% significance level). These results match very well the two-dimensional histogram shown in C, where the linear behavior goes from a clear inclined line to a vertical line at 33 min, indicating no more colocalization. E shows the least-square fits of the import rate of Rev into the nucleolus and the diffusion of CRM1 out of the nucleolus after injection of LMB versus the dissociation rate obtained in D, which clearly indicates that simply measuring the loss of CRM1 outside the nucleolus after injection of LMB had nothing to do with measuring the dissociation of Rev/CRM1.
Similar articles
- Nucleocytoplasmic transport of luciferase gene mRNA requires CRM1/Exportin1 and RanGTPase.
Kimura T, Hashimoto I, Nishikawa M, Yamada H. Kimura T, et al. Med Mol Morphol. 2009 Jun;42(2):70-81. doi: 10.1007/s00795-009-0441-3. Epub 2009 Jun 18. Med Mol Morphol. 2009. PMID: 19536614 - Kinetic and molecular analysis of nuclear export factor CRM1 association with its cargo in vivo.
Daelemans D, Costes SV, Lockett S, Pavlakis GN. Daelemans D, et al. Mol Cell Biol. 2005 Jan;25(2):728-39. doi: 10.1128/MCB.25.2.728-739.2005. Mol Cell Biol. 2005. PMID: 15632073 Free PMC article. - A regulatory role for CRM1 in the multi-directional trafficking of splicing snRNPs in the mammalian nucleus.
Sleeman J. Sleeman J. J Cell Sci. 2007 May 1;120(Pt 9):1540-50. doi: 10.1242/jcs.001529. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405816 - Atomic basis of CRM1-cargo recognition, release and inhibition.
Fung HY, Chook YM. Fung HY, et al. Semin Cancer Biol. 2014 Aug;27:52-61. doi: 10.1016/j.semcancer.2014.03.002. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631835 Free PMC article. Review. - Nuclear export of proteins and drug resistance in cancer.
Turner JG, Dawson J, Sullivan DM. Turner JG, et al. Biochem Pharmacol. 2012 Apr 15;83(8):1021-32. doi: 10.1016/j.bcp.2011.12.016. Epub 2011 Dec 20. Biochem Pharmacol. 2012. PMID: 22209898 Free PMC article. Review.
Cited by
- Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: clues on the mechanisms of venom-induced hemorrhage.
Herrera C, Escalante T, Voisin MB, Rucavado A, Morazán D, Macêdo JK, Calvete JJ, Sanz L, Nourshargh S, Gutiérrez JM, Fox JW. Herrera C, et al. PLoS Negl Trop Dis. 2015 Apr 24;9(4):e0003731. doi: 10.1371/journal.pntd.0003731. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25909592 Free PMC article. - Multiplexed protein profiling reveals spatial subcellular signaling networks.
Cai S, Hu T, Venkatesan M, Allam M, Schneider F, Ramalingam SS, Sun SY, Coskun AF. Cai S, et al. iScience. 2022 Aug 18;25(9):104980. doi: 10.1016/j.isci.2022.104980. eCollection 2022 Sep 16. iScience. 2022. PMID: 36093051 Free PMC article. - SPTBN1 Mediates the Cytoplasmic Constraint of PTTG1, Impairing Its Oncogenic Activity in Human Seminoma.
Teveroni E, Di Nicuolo F, Vergani E, Oliva A, Vodola EP, Bianchetti G, Maulucci G, De Spirito M, Cenci T, Pierconti F, Gulino G, Iavarone F, Urbani A, Milardi D, Pontecorvi A, Mancini F. Teveroni E, et al. Int J Mol Sci. 2023 Nov 29;24(23):16891. doi: 10.3390/ijms242316891. Int J Mol Sci. 2023. PMID: 38069214 Free PMC article. - Probing delivery of a lipid nanoparticle encapsulated self-amplifying mRNA vaccine using coherent Raman microscopy and multiphoton imaging.
Bera K, Rojas-Gómez RA, Mukherjee P, Snyder CE, Aksamitiene E, Alex A, Spillman DR Jr, Marjanovic M, Shabana A, Johnson R, Hood SR, Boppart SA. Bera K, et al. Sci Rep. 2024 Feb 22;14(1):4348. doi: 10.1038/s41598-024-54697-3. Sci Rep. 2024. PMID: 38388635 Free PMC article. - CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium.
Xavier AM, Ludwig RG, Nagai MH, de Almeida TJ, Watanabe HM, Hirata MY, Rosenstock TR, Papes F, Malnic B, Glezer I. Xavier AM, et al. Sci Rep. 2016 May 5;6:25507. doi: 10.1038/srep25507. Sci Rep. 2016. PMID: 27145700 Free PMC article.
References
- Akner, G., K. Mossberg, A. C. Wikstrom, K. G. Sundqvist, and J. A. Gustafsson. 1991. Evidence for colocalization of glucocorticoid receptor with cytoplasmic microtubules in human gingival fibroblasts, using two different monoclonal anti-GR antibodies, confocal laser scanning microscopy and image analysis. J. Steroid Biochem. Mol. Biol. 39:419–432. - PubMed
- Barbarese, E., D. E. Koppel, M. P. Deutscher, C. L. Smith, K. Ainger, F. Morgan, and J. H. Carson. 1995. Protein translation components are colocalized in granules in oligodendrocytes. J. Cell Sci. 108:2781–2790. - PubMed
- Checovich, W. J., R. E. Bolger, and T. Burke. 1995. Fluorescence polarization—a new tool for cell and molecular biology. Nature. 375:254–256. - PubMed
- Costes, S., E. Cho, M. Catalfamo, T. Karpova, J. McNally, P. Henkart, and S. Lockett. 2002. Automatic three-dimensional detection and quantification of colocalization. Proc. Microsc. Microanal. 8:S2-1040CD–S2-1048CD.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous