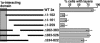Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication - PubMed (original) (raw)
Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication
Michael Schwartz et al. Proc Natl Acad Sci U S A. 2004.
Abstract
All positive-strand RNA [(+)RNA] viruses replicate their RNA on intracellular membranes, often in association with spherular invaginations of the target membrane. For brome mosaic virus, we previously showed that such spherules serve as compartments or mini-organelles for RNA replication and that their assembly, structure, and function have similarities to the replicative cores of retrovirus and double-stranded RNA virus virions. Some other (+)RNA viruses conduct RNA replication in association with individual or clustered double-membrane vesicles, appressed double membranes, or other structures whose possible relationships to the spherular invaginations are unclear. Here we show that modulating the relative levels and interactions of brome mosaic virus replication factors 1a and 2a polymerase (2apol) shifted the membrane rearrangements associated with RNA replication from small invaginated spherules to large, karmellae-like, multilayer stacks of appressed double membranes that supported RNA replication as efficiently as spherules. Spherules were induced by expressing 1a, which has functional similarities to retrovirus virion protein Gag, or 1a plus low levels of 2apol. Double-membrane layers were induced by 1a plus higher levels of 2apol and were suppressed by deleting the major 1a-interacting domain from 2apol. The stacked, double-membrane layers alternated with spaces that, like spherule interiors, were 50-60 nm wide, connected to the cytoplasm, and contained 1a and 2apol. These and other results suggest that seemingly diverse membrane rearrangements associated with RNA replication by varied (+)RNA viruses may represent topologically and functionally related structures formed by similar protein-protein and protein-membrane interactions and interconverted by altering the balances among those interactions.
Figures
Fig. 4.
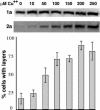
Increasing 2apol levels promotes 1a/2apol-mediated induction of double-membrane layers. Western analysis (Top and Middle) showing nearly constant 1a expression from the GAL1 promoter and linearly increasing 2apol expression from the copper-inducible CUP1 promoter in yeast grown in medium with the indicated levels of CuSO4. For each CuSO4 concentration, the histogram indicates the frequency of EM-visualized cell sections showing BMV-induced perinuclear double-membrane layers, relative to cells showing BMV-induced perinuclear spherules. The results show averages over two independent experiments, and in each experiment 150- to 200-cell sections with BMV-induced membrane rearrangements were scored for each indicated CuSO4 concentration. Standard error bars are indicated.
Fig. 5.
Deleting the N-proximal 1a-interacting domain of 2apol inhibits induction of double-membrane layers. Diagram of 2apol deletion derivatives with the indicated amino acids deleted. The shaded box indicates the 2apol segment directing high-affinity interaction with the 1a helicase-like domain (15, 16). The histogram indicates the frequency of EM-visualized cell sections that show BMV-induced perinuclear double-membrane layers relative to cells showing BMV-induced perinuclear spherules. The results show averages over two independent experiments, and in each experiment 150- to 200-cell sections with BMV-induced membrane rearrangements were scored for each 2apol deletion derivative. Standard error bars are indicated.
Fig. 1.
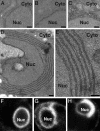
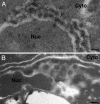
Two alternate membrane rearrangements are induced by 1a and 2apol. (A_–_E) Representative electron micrographs of yeast cells expressing no BMV components (A), GAL1 promoter-driven 2apol (B), GAL1 promoter-driven 1a and ADH1 promoter-driven 2apol (1aG+2aA) (C), or GAL1 promoter-driven 1a and 2aPol (1aG+2aG)(D and E). (F_–_H) Representative confocal fluorescence images of live yeast cells expressing Sec63p-GFP and no BMV components (F), 1aG+2aA (G), or 1aG+2aG (H). Nuc, nucleus; Cyto, cytoplasm. (Scale bars, 100 nm.)
Fig. 2.
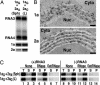
Double-membrane layers induced by 1a/2apol contain 1a and 2apol and support BMV RNA replication and 1a-induced membrane association and nuclease resistance of viral RNAs. (A) Northern and Western blot analyses of RNA3, RNA4, 1a and 2apol accumulation in 1aG+2aA yeast that contain perinuclear spherules (Sph), or 1aG+2aG yeast that contain double-membrane layers (L). (B) ImmunoGold EM localization of 1a (Upper) and 2apol (Lower) in 1aG+2aG yeast containing double-membrane layers. Replication factor 1a was localized with polyclonal anti-1a antiserum (17). Because anti-2a antibodies gave weak ImmunoGold labeling, 2apol was localized by using an anti-GFP monoclonal antibody (JL-8, Clontech) to detect a 2apol-GFP fusion that supports BMV RNA replication (16) and induced ultrastructural changes indistinguishable from WT 2apol (compare with Fig. 1_D_). Nuc, nucleus; Cyto, cytoplasm. (Scale bars, 100 nm.) (C) Northern blot analysis of cell fractionation extracts from 1aG+2aA yeast that contain spherules (upper row) and 1aG+2aG yeast that contain double-membrane layers (lower row). The analysis shows the distribution of (+)RNA3 and (–)RNA3 in total lysate (T), 20,000 × g membrane-depleted supernatant (S), or membrane-enriched pellet (P) fractions after one of the following treatments: no additional treatment (none), addition of 0.01 units/ml micrococcal nuclease/1 mM CaCl2 for 15 min at 30°C (RNase), or addition of 0.5% Nonidet P-40 for 15 min at 4°C followed by nuclease treatment (Det/RNase).
Fig. 3.
Isolated nuclei retain perinuclear, double-membrane layers, BMV RNA templates, and BMV-specific RNA-dependent RNA polymerase (RdRp) activity. (A) Representative electron micrograph of a nucleus isolated from 1aG+2aG yeast and bearing 1a/2apol-induced, double-membrane layers. (Scale bar, 100 nm.) (B) Northern blot analysis of (+)- and (–)-strand RNA3 and RNA4 and BMV-specific RNA-dependent RNA polymerase activity found in preparations of nuclei isolated from 1aG+2aA yeast that contain perinuclear spherules (Sph) or 1aG+2aG yeast containing double-membrane layers (L).
Fig. 6.
Underlying structure in the cytoplasm-connected spaces between double-membrane layers in 1aG+2aG yeast. See Results for further comments. Nuc, nucleus; Cyto, cytoplasm. (Scale bar, 100 nm.)
Fig. 7.
Models for 2apol modulation of 1a-dependent membrane rearrangements. See Discussion for further comments. (A) Invagination of a spherule (≈50 nm in diameter) into the outerperinuclear ER membrane by 1a formation of a membrane-enveloped, capsid-like shell with 1a recruitment of 2apol and viral RNA. Bold lines depict ER membranes and gray shading depicts the ER lumen. (B) High 2apol concentrations may promote zippering together of double-membrane layers by 1a–2a complexes. Replication factor 1a might participate as monomers or multimers (hexamers or pentamers). Self-interaction of 2apol might be direct or mediated by RNA or 2apol-interacting host proteins (33). As suggested by Fig. 6, spherule cores may be trapped in such layers. (C) High 2apol concentrations may block 1a–1a curvature needed to form spherules by steric hindrance between 2apol factors bound to adjacent 1a factors (left side), tending to restrict 1a to planar lattices (right side). (D) Between the stacked double-membrane layers, extended planar lattices of 1a with bound 2apol might provide a local replication complex environment similar to spherule interiors and may be closed by occasional regions of 1a curvature.
Similar articles
- Cowpea chlorotic mottle bromovirus replication proteins support template-selective RNA replication in Saccharomyces cerevisiae.
Sibert BS, Navine AK, Pennington J, Wang X, Ahlquist P. Sibert BS, et al. PLoS One. 2018 Dec 26;13(12):e0208743. doi: 10.1371/journal.pone.0208743. eCollection 2018. PLoS One. 2018. PMID: 30586378 Free PMC article. - Host acyl coenzyme A binding protein regulates replication complex assembly and activity of a positive-strand RNA virus.
Zhang J, Diaz A, Mao L, Ahlquist P, Wang X. Zhang J, et al. J Virol. 2012 May;86(9):5110-21. doi: 10.1128/JVI.06701-11. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345450 Free PMC article. - Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates.
Wang X, Lee WM, Watanabe T, Schwartz M, Janda M, Ahlquist P. Wang X, et al. J Virol. 2005 Nov;79(21):13747-58. doi: 10.1128/JVI.79.21.13747-13758.2005. J Virol. 2005. PMID: 16227294 Free PMC article. - Bromovirus-induced remodeling of host membranes during viral RNA replication.
Diaz A, Wang X. Diaz A, et al. Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review. - Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication.
Noueiry AO, Ahlquist P. Noueiry AO, et al. Annu Rev Phytopathol. 2003;41:77-98. doi: 10.1146/annurev.phyto.41.052002.095717. Epub 2003 Mar 10. Annu Rev Phytopathol. 2003. PMID: 12651962 Review.
Cited by
- Positive-strand RNA virus replication organelles at a glance.
Stancheva VG, Sanyal S. Stancheva VG, et al. J Cell Sci. 2024 Sep 1;137(17):jcs262164. doi: 10.1242/jcs.262164. Epub 2024 Sep 10. J Cell Sci. 2024. PMID: 39254430 Free PMC article. Review. - Evolution of RNA Viruses: Reasons for the Existence of Separate Plus, Minus, and Double-Strand Replication Strategies.
Park H, Higgs PG. Park H, et al. Viruses. 2024 Jul 5;16(7):1081. doi: 10.3390/v16071081. Viruses. 2024. PMID: 39066243 Free PMC article. - Exogenous and endogenous dsRNAs perceived by plant Dicer-like 4 protein in the RNAi-depleted cellular context.
Leonetti P, Consiglio A, Arendt D, Golbik RP, Rubino L, Gursinsky T, Behrens SE, Pantaleo V. Leonetti P, et al. Cell Mol Biol Lett. 2023 Aug 7;28(1):64. doi: 10.1186/s11658-023-00469-2. Cell Mol Biol Lett. 2023. PMID: 37550627 Free PMC article. - Endomembrane remodeling in SARS-CoV-2 infection.
Chen D, Zhao YG, Zhang H. Chen D, et al. Cell Insight. 2022 May 17;1(3):100031. doi: 10.1016/j.cellin.2022.100031. eCollection 2022 Jun. Cell Insight. 2022. PMID: 37193051 Free PMC article. Review. - Seed transmission of raspberry bushy dwarf virus is blocked in Nicotiana benthamiana plants by preventing virus entry into the embryo from the infected embryo sac and endosperm.
Isogai M, Yoshikoshi M, Seki K, Masuko-Suzuki H, Watanabe M, Matsuo K, Yaegashi H. Isogai M, et al. Arch Virol. 2023 Apr 12;168(5):138. doi: 10.1007/s00705-023-05767-w. Arch Virol. 2023. PMID: 37046148
References
- Hatta, T., Bullivant, S. & Matthews, R. E. (1973) J. Gen. Virol. 20, 37–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources