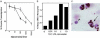Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques - PubMed (original) (raw)
Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques
Jaime Llodrá et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Some monocytes normally take up residence in tissues as sessile macrophages, but others differentiate into migratory cells resembling dendritic cells that emigrate to lymph nodes. In an in vitro model of a vessel wall, lipid mediators lysophosphatidic acid and platelet-activating factor, whose signals are implicated in promoting atherosclerosis, blocked conversion of monocytes into migratory cells and favored their retention in the subendothelium. In vivo studies revealed trafficking of monocyte-derived cells from atherosclerotic plaques during lesion regression, but little emigration was detected from progressive plaques. Thus, progression of atherosclerotic plaques may result not only from robust monocyte recruitment into arterial walls but also from reduced emigration of these cells from lesions.
Figures
Fig. 1.
Effect of PAF and LPA signals on the migratory fate of monocyte-derived cells in a model of a vessel wall. Carbamyl PAF (filled symbols) or 18:1 LPA (open symbols) was added to the culture medium at the concentrations shown. Reverse transmigration (a) and neutral lipid uptake (b) were quantified. (c) Arrows indicate a monocyte just beneath the endothelium that accumulated neutral lipid heavily, as assessed by staining with oil red O (ORO). Arrowhead shows a leukocyte that did not accumulate lipid. Shown is a representative experiment of three conducted, all with similar results.
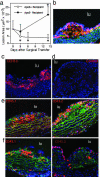
Fig. 2.
Assessment of methods and kinetics to study migration from lesions in vivo. (a) ApoE–/– male and female mice on a C57BL/6 Ly5.1 (CD45.1) congenic background were fed a Western-type diet from 4 to 19 weeks of age. Aortic arches from these donor mice were surgically implanted on the abdominal aorta of recipient mice (17). Each surgery included matched representatives of both genotypes, apoE+/+ and apoE–/–, for comparison between genotypes in side-by-side processing. Lesion area was quantified over a 2-week time course in an experimental design to compare outcomes in lesion size between apoE–/– and apoE+/+ recipients (five animals per time point) and to delineate the kinetics of regression in apoE+/+ recipients. * denotes significant change in lesion area from time 0, P < 0.02. Lesions examined at time 0 were stained with CD45.1 (b, red staining), CD68 (b, green staining that is in addition to autofluorescent green elastic lamina), and CD11c (c, red). Isotypematched control mAb did not stain the lesions (d). (e and f) Monocyte-derived cells from CD45.1 donor lesions (red staining, Left) or CD45.2 circulating monocytes of the recipient that were recruited to the lesion after surgical transfer (red staining, Right) were respectively identified in plaques 3 days after they were transferred to apoE+/+ or apoE–/– recipients. Counterstaining was with anti-CD68 mAb (green) and 4′,6-diamidino-2-phenylindole (blue). Green fluorescence was not recorded in c and d. lu denotes lumen of aorta.
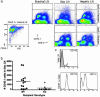
Fig. 3.
Tracing emigration from lesions to lymph nodes. (a) Flow cytometric evaluation was carried out on apoE–/– and apoE+/+ recipients 3 days after transplant. CD45.2 nontranplanted mouse lymph nodes were routinely stained with biotinylated anti-CD45.1 mAb and streptavidin-allophycocyanin to determine the extent of mAb cross-reactivity. This measure was used to delineate the position of the negative control line in each plot as shown. Staining of CD45.1+ control lymph nodes (LN) with anti-CD45.1 mAb established that the positively stained cells were uniformly detectable above the cross-reactive threshold (lower left flow plot). Staining was routinely conducted in single suspensions of iliac lymph nodes that drain the transferred aortic segments, the brachial lymph nodes as a representative distant nondraining lymph nodes, and the hepatic lymph node. Representative plots are shown, and each depict flow cytometric evaluation of the entire lymph node population. (b) Plot charts the total number of CD45.1+ cells observed in the iliac lymph nodes of individuals (each symbol, one individual) 3 days after aortic transfer into apoE+/+ (WT) or apoE–/– (KO) recipients. Differences in the number of migrated cells are statistically significant, P <0.03. (c) Evaluation of CD115 staining is shown in the iliac lymph nodes of a representative apoE+/+ recipient (bold line in Total LN histogram) compared with iliac lymph node of nontransplanted mouse (filled histogram). CD115 and CD11c staining in gated CD45.1 cells (lower histogram pair) is shown. Negative staining is observed in peaks that fall between 100 and 101 log.
Comment in
- The in and out of monocytes in atherosclerotic plaques: Balancing inflammation through migration.
Ludewig B, Laman JD. Ludewig B, et al. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11529-30. doi: 10.1073/pnas.0404612101. Epub 2004 Aug 3. Proc Natl Acad Sci U S A. 2004. PMID: 15292506 Free PMC article. Review. No abstract available.
Similar articles
- Atherosclerosis progression and monocyte emigration from plaque.
Packard RR, Shi GP. Packard RR, et al. Future Cardiol. 2006 Jul;2(4):415-8. doi: 10.2217/14796678.2.4.415. Future Cardiol. 2006. PMID: 19804177 - Oxidized monocyte-derived macrophages in aortic atherosclerotic lesion from apolipoprotein E-deficient mice and from human carotid artery contain lipid peroxides and oxysterols.
Maor I, Kaplan M, Hayek T, Vaya J, Hoffman A, Aviram M. Maor I, et al. Biochem Biophys Res Commun. 2000 Mar 24;269(3):775-80. doi: 10.1006/bbrc.2000.2359. Biochem Biophys Res Commun. 2000. PMID: 10720491 - Monocytes/macrophages in atherosclerosis.
Plenz G, Robenek H. Plenz G, et al. Eur Cytokine Netw. 1998 Dec;9(4):701-3. Eur Cytokine Netw. 1998. PMID: 9889421 Review. - The in and out of monocytes in atherosclerotic plaques: Balancing inflammation through migration.
Ludewig B, Laman JD. Ludewig B, et al. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11529-30. doi: 10.1073/pnas.0404612101. Epub 2004 Aug 3. Proc Natl Acad Sci U S A. 2004. PMID: 15292506 Free PMC article. Review. No abstract available.
Cited by
- The complex fate in plasma of gadolinium incorporated into high-density lipoproteins used for magnetic imaging of atherosclerotic plaques.
Barazza A, Blachford C, Even-Or O, Joaquin VA, Briley-Saebo KC, Chen W, Jiang XC, Mulder WJ, Cormode DP, Fayad ZA, Fisher EA. Barazza A, et al. Bioconjug Chem. 2013 Jun 19;24(6):1039-48. doi: 10.1021/bc400105j. Epub 2013 May 10. Bioconjug Chem. 2013. PMID: 23617731 Free PMC article. - Executable models of immune signaling pathways in HIV-associated atherosclerosis.
Palshikar MG, Palli R, Tyrell A, Maggirwar S, Schifitto G, Singh MV, Thakar J. Palshikar MG, et al. NPJ Syst Biol Appl. 2022 Sep 21;8(1):35. doi: 10.1038/s41540-022-00246-5. NPJ Syst Biol Appl. 2022. PMID: 36131068 Free PMC article. - High density lipoprotein and metabolic disease: Potential benefits of restoring its functional properties.
Klancic T, Woodward L, Hofmann SM, Fisher EA. Klancic T, et al. Mol Metab. 2016 Mar 18;5(5):321-327. doi: 10.1016/j.molmet.2016.03.001. eCollection 2016 May. Mol Metab. 2016. PMID: 27110484 Free PMC article. Review. - Lipoxins modulate neutrophil oxidative burst, integrin expression and lymphatic transmigration differentially in human health and atherosclerosis.
Kraft JD, Blomgran R, Bergström I, Soták M, Clark M, Rani A, Rajan MR, Dalli J, Nyström S, Quiding-Järbrink M, Bromberg J, Skoog P, Börgeson E. Kraft JD, et al. FASEB J. 2022 Mar;36(3):e22173. doi: 10.1096/fj.202101219RR. FASEB J. 2022. PMID: 35104001 Free PMC article. Clinical Trial. - Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages.
Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Smythies LE, et al. Am J Physiol Cell Physiol. 2010 Jun;298(6):C1538-48. doi: 10.1152/ajpcell.00467.2009. Epub 2010 Mar 10. Am J Physiol Cell Physiol. 2010. PMID: 20219948 Free PMC article.
References
- Glass, C. K. & Witztum, J. L. (2001) Cell 104, 503–516. - PubMed
- Osterud, B. & Bjorklid, E. (2003) Physiol. Rev. 83, 1069–1112. - PubMed
- Bobryshev, Y. V. (2000) Curr. Opin. Lipidol. 11, 511–517. - PubMed
- Van Furth, R. (1988) in Inflammation: Basic Principles and Clinical Correlates, eds. Gallin, J. I., Goldstein, I. M. & Snyderman, R. (Raven, New York), pp. 281–295.
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI049653/AI/NIAID NIH HHS/United States
- R01 HL069446/HL/NHLBI NIH HHS/United States
- R01 AI049653/AI/NIAID NIH HHS/United States
- AI49653/AI/NIAID NIH HHS/United States
- HL69446/HL/NHLBI NIH HHS/United States
- HL61814/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources