NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity - PubMed (original) (raw)
NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity
Shuichi Fujioka et al. Mol Cell Biol. 2004 Sep.
Abstract
Nuclear factor kappaB (NF-kappaB) and activator protein 1 (AP-1) transcription factors regulate many important biological and pathological processes. Activation of NF-kappaB is regulated by the inducible phosphorylation of NF-kappaB inhibitor IkappaB by IkappaB kinase. In contrast, Fos, a key component of AP-1, is primarily transcriptionally regulated by serum responsive factors (SRFs) and ternary complex factors (TCFs). Despite these different regulatory mechanisms, there is an intriguing possibility that NF-kappaB and AP-1 may modulate each other, thus expanding the scope of these two rapidly inducible transcription factors. To determine whether NF-kappaB activity is involved in the regulation of fos expression in response to various stimuli, we analyzed activity of AP-1 and expression of fos, fosB, fra-1, fra-2, jun, junB, and junD, as well as AP-1 downstream target gene VEGF, using MDAPanc-28 and MDAPanc-28/IkappaBalphaM pancreatic tumor cells and wild-type, IKK1-/-, and IKK2-/- murine embryonic fibroblast cells. Our results show that elk-1, a member of TCFs, is one of the NF-kappaB downstream target genes. Inhibition of NF-kappaB activity greatly decreased expression of elk-1. Consequently, the reduced level of activated Elk-1 protein by extracellular signal-regulated kinase impeded constitutive, serum-, and superoxide-inducible c-fos expression. Thus, our study revealed a distinct and essential role of NF-kappaB in participating in the regulation of elk-1, c-fos, and VEGF expression.
Copyright 2004 American Society for Microbiology
Figures
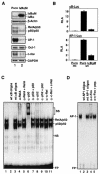
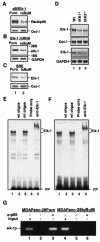
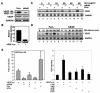
FIG. 1.
Suppression of constitutive NF-κB activity inhibits overexpression of c-fos and AP-1 activity. (A) The expression of IκBα and IκBαM in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells was determined by Western blot analysis with the cytoplasmic protein extracts using β-actin as loading controls. The nuclear protein extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells were subjected to EMSA with NF-κB, AP-1, and the Oct-1 probe as a control. Expression of c-fos in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells was determined by Northern blot analysis with GAPDH as the loading control. (B) Luciferase reporter gene assays for NF-κB and AP-1 activities were performed with wild-type (wt) and mutant (mt) NF-κB and AP-1 luciferase reporter gene constructs transiently transfected into MDAPanc-28/Puro and MDAPanc-28/IκBαM cells. (C and D) Competition and supershift assays were performed with EMSA to determine the specificity of the constitutive RelA/p50 NF-κB and AP-1 DNA binding activities. Ten micrograms of nuclear extract from MDAPanc-28/Puro cells was incubated with a 50× excess of unlabeled wild-type NF-κB or AP-1 probe (C and D, lane 2), mutant NF-κB or AP-1 probe (C and D, lane 3), and anti-NF-κB and anti-Fos antibodies (α-), with and without control peptides, as indicated. FP, free probe.
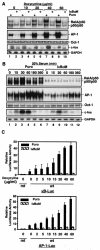
FIG. 2.
Induction of AP-1 activity and fos expression by doxycycline-induced ROS requires NF-κB activation, and serum-induced AP-1 activity and fos expression are partially dependent on NF-κB activation. (A) EMSAs were performed to determine NF-κB and AP-1 activity by using the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with increasing doses ofdoxycycline as indicated for 24 h. An Oct-1 probe was used as a control for quality and quantity of cell extracts. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline for the indicated dosages and analyzed by Northern blotting with a human c-fos cDNA probe, and the same blot was rehybridized to a gapdh cDNA probe. (B) Inhibition of serum-induced activation of NF-κB and AP-1 was determined by using EMSAs with the nuclear extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with 20% serum for the indicated times after 48 h of serum deprivation. An Oct-1 probe was used as a control for quality and quantity of cell extracts. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with 20% serum for the indicated time and analyzed by Northern blotting with a human c-fos cDNA probe, and the same blot was rehybridized to a gapdh cDNA probe. (C) IκBαM-mediated inhibition of the doxycycline-induced activation of NF-κB and AP-1 activities was determined with wild-type (wt) and mutant (mt) NF-κB and AP-1 luciferase reporter gene constructs transiently transfected into MDAPanc-28/Puro and MDAPanc-28/IκBαM cells.
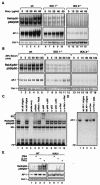
FIG. 3.
Doxycycline- and serum-inducible NF-κB and AP-1 activities were inhibited in IKK1−/− and IKK2−/− MEF cells. Wild-type (wt), IKK1−/−, and IKK2−/− MEF cells were stimulated with doxycycline as indicated for 24 h (A), and wild-type, IKK1−/−, and IKK2−/− MEF cellswere stimulated with 20% serum for the indicated times after 48 h of serum starvation (B). Nuclear extracts were isolated and subjected to EMSA with NF-κB and AP-1 probes. (C and D) Competition and supershift assays were performed with EMSA to determine the specificity of the doxycycline-induced RelA/p50 NF-κB and AP-1 DNA binding activities. Ten micrograms of nuclear protein from doxycycline-stimulated wild-type MEF cells with a 50× excess of unlabeled wild-type NF-κB or AP-1 probe (C and D, lane 2) or mutant NF-κB or AP-1 probe (C and D, lane 3), along with anti-NF-κB and anti-Fos antibodies (α-), with and without control peptides, as indicated. FP, free probe. (E) Wild-type (WT) and IKK2−/− MEF cells were stimulated with PMA (50 μg/ml), IL-1α (10 ng/ml), and TNF-α (10 ng/ml) for 30 min, and EMSAs with NF-κB, AP-1, and Oct-1 probes were performed.
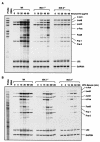
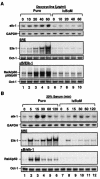
FIG. 4.
Doxycycline- and serum-inducible expressions of the AP-1 family members were determined in wild-type (Wt), IKK1−/−, and IKK2−/− MEF cells. RNA was isolated from wild-type, IKK1−/−, and IKK2−/− MEF cells stimulated with either doxycycline or 20% serum for the indicated doses and times. RNase protection assays were carried out with the Riboquant multiprobe protection assay system to determine doxycycline (A)- and serum stimulation (B)-induced expression of AP-1 components in wild-type, IKK1−/−, and IKK2−/− MEF cells as indicated.
FIG. 5.
NF-κB activity regulates elk-1 expression. (A) EMSAs were performed with the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells to determine the NF-κB binding activity to the NF-κB motif on the elk-1 promoter with an Oct-1 probe as a control. (B) elk-1 mRNA expression was determined by Northern blot analysis with RNA from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells with elk-1 cDNA and gapdh probe as a loading control. (C) Elk-1 DNA binding activity on SRE from the c-fos promoter was determined by EMSA with the nuclear extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells (A) and an Oct-1 probe as a control. (D) EMSAs were performed with the nuclear extracts isolatedfrom wild-type (Wt), IKK1−/−, and IKK2−/− MEF cells to determine the Elk-1 DNA binding activity with the SRE probe. An Oct-1 probe was used as a control for loading. The expression of elk-1 was determined by Northern blot analysis with the RNA isolated from wild-type, IKK1−/−, and IKK2−/− MEF cells with mouse elk-1 cDNA and gapdh probes. (E and F) Elk-1 activity in MDAPanc-28/Puro (E) and wild-type MEF (F) cells was determined by competition and supershift assay with 10 μg of nuclear extract with a 50× excess of unlabeled wild-type and mutant Elk-1 probe (lanes 2 and 3) and anti-Elk-1 antibody as indicated. FP, free probe. (G) ChIP assays were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells with and without anti-p65 (NF-κB) antibody as indicated.
FIG. 6.
NF-κB activity is essential for doxycycline- and serum-induced elk-1 expression. (A) Doxycycline- and (B) serum-induced NF-κB-dependent induction of elk-1 expression was determined by Northern blot analysis. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline at the indicated doses and 20% serum for the indicated times and hybridized with a human elk-1 cDNA probe and rehybridized to a gapdh cDNA probe. (A) Doxycycline (A)- and serum (B)-induced NF-κB-dependent activation of Elk-1 was determined by EMSAs with NF-κB and SRE probes and the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline as indicated for 24 h or with 20% serum for the indicated times after 48 h of serum withdrawal. An Oct-1 probe was used as a control for quality and quantity of cell extracts.
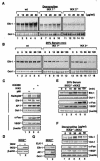
FIG. 7.
IKK is essential for doxycycline- and serum-induced elk-1 expression. Wild-type (wt), IKK1−/−, and IKK2−/− MEF cells were stimulated with doxycycline at the indicated doses for 24 h (A) or with 20% serum for the indicated times after 48 h of serum withdrawal (B). Nuclear extracts were isolated and subjected to EMSA with an SRE probe. (C) Nuclear and whole-cell extracts were isolated from the IKK2 (S177, 181E), IKK2, and p65 (NF-κB) transfectants and analyzed by EMSA with NF-κB, Elk-1, AP-1, and Oct-1 probes and Western blotting with anti-cFos, VEGF, and β-actin antibodies as indicated. (D) The protein extracts of the IKK2- or vector-transfected IKK2−/− clone were analyzed for the expression of transfected IKK2 in Western blotting with β-actin as loading controls. (E) IKK2-reconstituted cells from panel D were stimulated with 20% serum for the indicated times after 48 h of serum starvation. (F) IKK2-reconstituted cells from panel D were stimulated with doxycycline at the indicated doses for 24 h. Nuclear extracts from panels E and F were isolated and analyzed by EMSA with NF-κB, AP-1, and Oct-1 probes, and whole-cell extracts from both panels E and F were subjected to Western blot analysis with anti-c-Fos, -VEGF, and -β-actin antibodies as indicated. (G) The nuclear extracts from an IKK2−/− clone transfected with an expression plasmid encoding Elk-1 were analyzed by EMSA with SRE and AP-1 probes and with Oct-1 probe as a loading control.
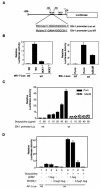
FIG. 8.
NF-κB regulates the elk-1 promoter, and a dominant mutant Elk-1 inhibits AP-1 activity. (A) elk-1 promoter and elk-1 promoter luciferase reporter gene constructs with wild-type (wt) and mutant (MT) κBelk-1 sequences indicated. (B) Luciferase reporter gene assays for the basal activity of elk-1 promoter constructs with wild-type (Wt) κB and mutant (mt) κB binding sites were performed with wild-type, IKK1−/−, and IKK2−/− MEF cells as indicated. (C) Luciferase reporter gene assays for the inducible of elk-1 promoter activity were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells cotransfected with elk-1 promoter reporter gene constructs with wild-type and mutant κB binding sites and p-TK Renilla luciferase and then stimulated with doxycycline as indicated. (D) MDAPanc-28/Puro and MDAPanc-28/IκBαM cells were cotransfected either with the indicated amount of pCMV expression vector encoding the mutant Elk-1 or control pCMV vector, AP-1-luciferase reporter gene construct, and p-TK Renilla luciferase in the absence or presence of doxycycline stimulation. The transient transfections in panels B, C, and D were performed by the lipotransfection method (FuGENE 6; Roche). The activities of both firefly luciferase and Renilla luciferase were determined with the Promega dual-luciferase reporter assay system. The luciferase activities were normalized to the Renilla luciferase activity of the internal control. Data represent the mean ± standard error from three different experiments performed in triplicate.
FIG. 9.
NF-κB-mediated AP-1 activity regulates VEGF expression. (A) Western blot and Northern analyses of VEGF expression in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells as indicated. (B) Luciferase reporter gene assays for the activity of a VEGF promoter construct were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells as indicated. (C and D) Northern blot analysis of VEGF expression in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with serum at various time intervals and doxycycline at different doses. (E) Luciferase reporter gene assays for the activity of a VEGF promoter construct were performed with the expression vectors encoding IκBαM, a dominant-negative Fos mutant (ΔFos), Fos, FosB, JunB, JunD, and c-Jun as indicated. The activities of both firefly luciferase and Renilla luciferase were determined by using the Promega dual-luciferase reporter assay system (Promega). The luciferase activities were normalized to the Renilla luciferase activity of the internal control. Data represent the mean ± standard error from three different experiments performed in triplicate.
Similar articles
- Induction of the RelB NF-kappaB subunit by the cytomegalovirus IE1 protein is mediated via Jun kinase and c-Jun/Fra-2 AP-1 complexes.
Wang X, Sonenshein GE. Wang X, et al. J Virol. 2005 Jan;79(1):95-105. doi: 10.1128/JVI.79.1.95-105.2005. J Virol. 2005. PMID: 15596805 Free PMC article. - Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation.
Gallo A, Cuozzo C, Esposito I, Maggiolini M, Bonofiglio D, Vivacqua A, Garramone M, Weiss C, Bohmann D, Musti AM. Gallo A, et al. Oncogene. 2002 Sep 19;21(42):6434-45. doi: 10.1038/sj.onc.1205822. Oncogene. 2002. PMID: 12226747 - Regulation of the MIR155 host gene in physiological and pathological processes.
Elton TS, Selemon H, Elton SM, Parinandi NL. Elton TS, et al. Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
- Induction of NUPR1 and AP‑1 contributes to the carcinogenic potential of nickel.
Murphy A, Roy N, Sun H, Jin C, Costa M. Murphy A, et al. Oncol Rep. 2021 Apr;45(4):41. doi: 10.3892/or.2021.7992. Epub 2021 Mar 2. Oncol Rep. 2021. PMID: 33649793 Free PMC article. - The nexus between VEGF and NFκB orchestrates a hypoxia-independent neovasculogenesis.
DeNiro M, Al-Mohanna FH, Alsmadi O, Al-Mohanna FA. DeNiro M, et al. PLoS One. 2013;8(3):e59021. doi: 10.1371/journal.pone.0059021. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533599 Free PMC article. Retracted. - Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases.
Leyva-López N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Leyva-López N, et al. Int J Mol Sci. 2016 Jun 9;17(6):921. doi: 10.3390/ijms17060921. Int J Mol Sci. 2016. PMID: 27294919 Free PMC article. Review. - TNFAIP1 interacts with KCTD10 to promote the degradation of KCTD10 proteins and inhibit the transcriptional activities of NF-κB and AP-1.
Hu X, Yan F, Wang F, Yang Z, Xiao L, Li L, Xiang S, Zhou J, Ding X, Zhang J. Hu X, et al. Mol Biol Rep. 2012 Nov;39(11):9911-9. doi: 10.1007/s11033-012-1858-7. Epub 2012 Jul 19. Mol Biol Rep. 2012. PMID: 22810651 - Tip110/SART3-Mediated Regulation of NF-κB Activity by Targeting IκBα Stability Through USP15.
Timani KA, Rezaei S, Whitmill A, Liu Y, He JJ. Timani KA, et al. Front Oncol. 2022 Apr 21;12:843157. doi: 10.3389/fonc.2022.843157. eCollection 2022. Front Oncol. 2022. PMID: 35530338 Free PMC article.
References
- Abate, C., L. Patel, F. J. Rauscher III, and T. Curran. 1990. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249:1157-1161. - PubMed
- Abo, A., E. Pick, A. Hall, N. Totty, C. G. Teahan, and A. W. Segal. 1991. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668-670. - PubMed
- Baeuerle, P. A., and D. Baltimore. 1988. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 53:211-217. - PubMed
- Baeuerle, P. A., and D. Baltimore. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540-546. - PubMed
- Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous