Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation - PubMed (original) (raw)
Comparative Study
. 2004 Sep 28;101(39):14294-9.
doi: 10.1073/pnas.0405968101. Epub 2004 Sep 20.
Affiliations
- PMID: 15381765
- PMCID: PMC521149
- DOI: 10.1073/pnas.0405968101
Comparative Study
Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation
Henrik Mouritsen et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Migratory birds can use a magnetic compass for orientation during their migratory journeys covering thousands of kilometers. But how do they sense the reference direction provided by the Earth's magnetic field? Behavioral evidence and theoretical considerations have suggested that radical-pair processes in differently oriented, light-sensitive molecules of the retina could enable migratory birds to perceive the magnetic field as visual patterns. The cryptochromes (CRYs) have been suggested as the most likely candidate class of molecules, but do CRYs exist in the retina of migratory birds? Here, we show that at least one CRY1 and one CRY2 exist in the retina of migratory garden warblers and that garden-warbler CRY1 (gwCRY1) is cytosolic. We also show that gwCRY1 is concentrated in specific cells, particularly in ganglion cells and in large displaced ganglion cells, which also showed high levels of neuronal activity at night, when our garden warblers performed magnetic orientation. In addition, there seem to be striking differences in CRY1 expression between migratory and nonmigratory songbirds at night. The difference in CRY1 expression between migrants and nonmigrants is particularly pronounced in the large displaced ganglion cells known to project exclusively to a brain area where magnetically sensitive neurons have been reported. Consequently, cytosolic gwCRY1 is well placed to possibly be the primary magnetic-sensory molecule required for light-mediated magnetoreception.
Figures
Fig. 1.
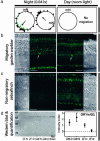
CRY protein expression in the retina of migratory garden warblers and nonmigratory zebra finches. (a) Magnetic orientation of garden warblers. Each data point indicates the mean orientation of one individual. The arrows indicate group mean vector lengths (r). The orientation of the night-birds (Night) from which retinas were collected (green symbols) were highly oriented in the normal migratory direction (n = 11, mean = 223°, r = 0.77, and P < 0.001). The red dot represents the night bird whose retina is shown in b. During the day, the birds do not show orientation behavior (Day). mN, Magnetic North. (b) Immunohistochemical staining of CRY1 protein during nocturnal magnetic orientation (Left) and during the day (Right) in migratory garden warblers (images from the same slide taken with identical settings). Large displaced ganglion cells marked with arrow. (c) Immunohistochemical staining of CRY1 protein during the night (Left) and day (Right) in nonmigratory zebra finches (images from same slide taken with identical settings). Labeling of photoreceptor outer segments and Müller cell end-feet in b and c is unspecific. (d) Example of Western blot analysis confirming specificity of the antibody and quantification of CRY1 expression in the ganglion cell layer. For definition of intensity index, see Materials and Methods. Dashed line indicates unspecific background level of expression. GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer. (Scale bar, 40 μm.)
Fig. 2.
Detection of a CRY1 transcript with the expected size of ≈642 bp in the left and right retinas of garden warbler (lower sequence). The deduced amino acid sequence for this fragment reveals 91% identity with the specific C-terminal region of chicken CRY1 (upper sequence). The FAD-binding domain thought to be involved in radical-pair reactions is shown in bold. Upper left, 5′ end; lower right, 3′ end.
Fig. 3.
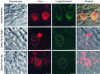
Colocalization of CRY1 and neuronal-activity markers in the same 300-nm-thick retina slice from a magnetically orienting garden warbler. (a) Example of Western blot analysis confirming specificity of the antibody and quantification of c-Fos expression in the ganglion cell layer. For definition of intensity index, see Materials and Methods. Dashed line indicates unspecific background level of expression. (b and c) Immunohistochemical double labeling of CRY1 (cytosolic) with the neuronal-activity markers c-Fos (b; cytosolic and nucleic) and ZENK (c; exclusively nucleic; only the ganglion cell layer is shown) reveal that CRYs are found in night-active ganglion cells and in large displaced ganglion cells of the INL (arrows). (Scale bar, 40 μm.) GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Fig. 4.
High magnification confocal images confirm the colocalization of CRY1 with the neuronal-activity marker c-Fos in large displaced ganglion cells in the garden warbler [during both night (shown) and day (data not shown)]. In contrast, the large displaced ganglion cells never express CRY1 in zebra finches, neither during the night nor during the day, when both active (c-Fos-positive, as shown) and inactive (c-Fos-negative) large displaced ganglion cells occur in zebra finches.
Similar articles
- Cryptochrome expression in the eye of migratory birds depends on their migratory status.
Fusani L, Bertolucci C, Frigato E, Foà A. Fusani L, et al. J Exp Biol. 2014 Mar 15;217(Pt 6):918-23. doi: 10.1242/jeb.096479. J Exp Biol. 2014. PMID: 24622895 - Orientation of birds in radiofrequency fields in the absence of the Earth's magnetic field: a possible test for the radical pair mechanism of magnetoreception.
Luo J, Benjamin P, Gerhards L, Hogben HJ, Hore PJ. Luo J, et al. J R Soc Interface. 2024 Aug;21(217):20240133. doi: 10.1098/rsif.2024.0133. Epub 2024 Aug 7. J R Soc Interface. 2024. PMID: 39110232 Free PMC article. - Double-Cone Localization and Seasonal Expression Pattern Suggest a Role in Magnetoreception for European Robin Cryptochrome 4.
Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch KW, Solov'yov IA, Mouritsen H. Günther A, et al. Curr Biol. 2018 Jan 22;28(2):211-223.e4. doi: 10.1016/j.cub.2017.12.003. Epub 2018 Jan 4. Curr Biol. 2018. PMID: 29307554 - The magnetic map sense and its use in fine-tuning the migration programme of birds.
Heyers D, Elbers D, Bulte M, Bairlein F, Mouritsen H. Heyers D, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Jul;203(6-7):491-497. doi: 10.1007/s00359-017-1164-x. Epub 2017 Apr 1. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28365788 Review. - The Radical-Pair Mechanism of Magnetoreception.
Hore PJ, Mouritsen H. Hore PJ, et al. Annu Rev Biophys. 2016 Jul 5;45:299-344. doi: 10.1146/annurev-biophys-032116-094545. Epub 2016 May 16. Annu Rev Biophys. 2016. PMID: 27216936 Review.
Cited by
- A robust synthesis of 7,8-didemethyl-8-hydroxy-5-deazariboflavin.
Bender M, Mouritsen H, Christoffers J. Bender M, et al. Beilstein J Org Chem. 2016 May 6;12:912-7. doi: 10.3762/bjoc.12.89. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27340481 Free PMC article. - Magnetoreception in plants.
Galland P, Pazur A. Galland P, et al. J Plant Res. 2005 Dec;118(6):371-89. doi: 10.1007/s10265-005-0246-y. Epub 2005 Nov 9. J Plant Res. 2005. PMID: 16283069 Review. - Functional motifs in the (6-4) photolyase crystal structure make a comparative framework for DNA repair photolyases and clock cryptochromes.
Hitomi K, DiTacchio L, Arvai AS, Yamamoto J, Kim ST, Todo T, Tainer JA, Iwai S, Panda S, Getzoff ED. Hitomi K, et al. Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):6962-7. doi: 10.1073/pnas.0809180106. Epub 2009 Apr 9. Proc Natl Acad Sci U S A. 2009. PMID: 19359474 Free PMC article. - Radical-pair-based magnetoreception in birds: radio-frequency experiments and the role of cryptochrome.
Nießner C, Winklhofer M. Nießner C, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Jul;203(6-7):499-507. doi: 10.1007/s00359-017-1189-1. Epub 2017 Jun 13. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28612234 Free PMC article. - The symbiotic magnetic-sensing hypothesis: do Magnetotactic Bacteria underlie the magnetic sensing capability of animals?
Natan E, Vortman Y. Natan E, et al. Mov Ecol. 2017 Oct 23;5:22. doi: 10.1186/s40462-017-0113-1. eCollection 2017. Mov Ecol. 2017. PMID: 29085642 Free PMC article.
References
- Becker, G. & Speck, U. (1964) Z. Vergl. Physiol. 49, 301–340.
- Lindauer, M. & Martin, H. (1968) Z. Vergl. Physiol. 60, 219–243.
- Wiltschko, W. (1968) Z. Tierpsychol. 25, 537–558. - PubMed
- Wiltschko, W. & Wiltschko, R. (1972) Science 176, 62–64. - PubMed
- Wiltschko, R. & Wiltschko, W. (1995) Magnetic Orientation in Animals (Springer, Berlin).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources