The origin of the parathyroid gland - PubMed (original) (raw)
The origin of the parathyroid gland
Masataka Okabe et al. Proc Natl Acad Sci U S A. 2004.
Abstract
It has long been held that the parathyroid glands and parathyroid hormone evolved with the emergence of the tetrapods, reflecting a need for new controls on calcium homeostasis in terrestrial, rather than aquatic, environments. Developmentally, the parathyroid gland is derived from the pharyngeal pouch endoderm, and studies in mice have shown that its formation is under the control of a key regulatory gene, Gcm-2. We have used a phylogenetic analysis of Gcm-2 to probe the evolutionary origins of the parathyroid gland. We show that in chicks, as in mice, Gcm-2 is expressed in the pharyngeal pouches and the forming parathyroid gland. We find that Gcm-2 is present not only in tetrapods but also in teleosts and chondrichthyans, and that in these species, Gcm-2 is expressed within the pharyngeal pouches and internal gill buds that derive from them in zebrafish (Danio rerio), a teleost, and dogfish (Scyliorhinus canicula), a chondrichthyan. We further demonstrate that Gcm-2 is required for the formation of the internal gill buds in zebrafish. We also have identified parathyroid hormone 1/2-encoding genes in fish and show that these genes are expressed by the gills. We further show that the gills express the calcium-sensing receptor, which is used in tetrapods to monitor serum calcium levels. These results indicate that the tetrapod parathyroid gland and the gills of fish are evolutionarily related structures, and that the parathyroid likely came into being as a result of the transformation of the gills during tetrapod evolution.
Figures
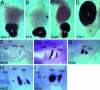
Fig. 1.
Expression of Gcm-2 in the parathyroid gland and the pharyngeal pouches in the chick. (A_–_D) Whole-mount in situ hybridization of chick E11 thyroid (T) and parathyroid (P) glands for the following probes: Gcm-2 (A), PTH (B), CasR (C), and TTF-1 (D). Gcm-2 can be seen to be expressed in two round masses, the parathyroid glands, which are adjacent to the thyroid, which expresses TTF-1. The parathyroid glands additionally express PTH and CasR. (E_–_I) Expression of Gcm-2 in chicken embryos, staged as described in ref. . In these micrographs, anterior is to the left and ventral is to the bottom. OV, otic vesicle; pp, pharyngeal pouch; II–IV, pharyngeal arches. Expression of Gcm-2 starts in the third pharyngeal pouch at stage 18 (E), and then, as development proceeds, expression is also evident in the fourth pharyngeal pouch and additionally weakly in the second pouch (F). At stage 24, expression in the third and fourth pouches is concentrated in a region dorsal of the pharyngeal pouches and is lost from the second pouch (G). In Vibratome sections of stage-22 embryos (H), it is clear that Gcm-2 expression is localized to the pharyngeal endoderm and that, by stage 24, the region of the pharyngeal endoderm expressing Gcm-2 has thickened and given rise to round masses that are the parathyroid gland rudiments of the third and fourth pouches (I).
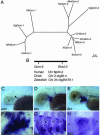
Fig. 2.
Phylogenetic analysis of the distribution of Gcm-2 and its expression in teleost (zebrafish) and chondrichthyan (dogfish) species. (A) A phylogenetic tree of vertebrate Gcm genes based on
clustal
analysis. Dr, zebrafish (Danio rerio); Gg, chicken (Gallus gallus); Hs, human (Homo sapiens); Mm, mouse (Mus musculus); Nf, Australian lungfish (Neoceratodus forsteri); Om, rainbow trout (Oncorhynchus mykiss); Sc, dogfish (Scyliorhinus canicula); Xl, Xenopus (Xenopus laevis). Branch lengths are in units of number of amino acid substitutions per site. (B) Schematic depicting the conserved linkage between Gcm2 and Elovl2 in humans on chromosome 6p24.2, in chickens on chromosome 2 ctg20.4, and in zebrafish on chromosome 24 ctg25479.1. (C_–_F) Gcm-2 expression in zebrafish embryos. In these micrographs, anterior is to the left and ventral is to the bottom. Gcm-2 initiates expression in the second pharyngeal pouch in early 3-day-old larval fish (indicated by arrowhead in C). Subsequently, Gcm-2 is expressed sequentially in the more posterior pouches (D), and, by day 4, Gcm-2 is expressed in all of the pouches (E). It is also apparent by day 4 that Gcm-2 is expressed in the developing internal gill buds emerging from the pharyngeal pouches (F). (G and H) Gcm-2 expression in dogfish embryos. This gene is expressed in the internal gill buds protruding from the pharyngeal pouches in stage-27 dogfish embryos (17). The pharyngeal arches are numbered II–VI.
Fig. 3.
Gcm-2 is required for the elaboration of the internal gill buds from the pharyngeal pouches in zebrafish. Zebrafish embryos were injected at the one-cell stage with either control or antisense Gcm-2 MOs. The embryos were then analyzed at day 5 for the presence of internal gill buds. (A_–_C) Five-day-old zebrafish larva injected with control MO. (A) Nomarski view of the pharyngeal region of a day-5 embryo injected with the control MO. The internal gill buds protruding from the pharyngeal pouches are clearly evident (arrowheads). (B) Embryo injected with control MO hybridized for Gcm-2. _Gcm-2_-expressing internal gill buds can be clearly seen protruding from the pharyngeal pouches. (C) Embryo injected with control MO, showing normal pharyngeal pouch formation as judged by Pax-9a expression. Each pharyngeal pouch is indicated by an arrowhead. (D_–_F) Five-day-old zebrafish larva injected with Gcm-2 antisense MO. (D) Nomarski view of the pharyngeal region of a E5 embryo injected with the antisense Gcm-2 MO. There are no internal gill buds protruding from the pharyngeal pouches. (E) Embryo injected with the antisense Gcm-2 MO hybridized for Gcm-2. There are no _Gcm-2_-expressing internal gill buds protruding from the pharyngeal pouches. (F) Embryo injected with the antisense Gcm-2 MO, showing normal pharyngeal pouch formation as judged by Pax-9a expression. Each pharyngeal pouch is indicated by an arrowhead. EY, eye; YK, yolk. Anterior is to left and ventral is to the bottom.
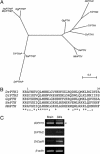
Fig. 4.
PTH genes in zebrafish. (A) A phylogenetic tree of PTH and PTHrP genes in vertebrates. Dr, zebrafish (Danio rerio); Gg, chicken (Gallus gallus); Hs, human (Homo sapiens); Mm, mouse (Mus musculus); Sa, seabream (Sparus aurata); Tr, fugu (Takifugu rubripes). (B) Comparison of the partial peptide sequences of zebrafish PTH and amniote PTH. The N-terminal 34 aa of mature human PTH peptide are sufficient for the biological activity of PTH. This alignment includes the N-terminal amino acids (1–34) with 2 aa before the final proteolytic cleavage site. (C) Gills in adult zebrafish express both the PTH genes and CasR. PTH and CasR were amplified from adult brain and gills by RT-PCR. Arrowhead indicates cDNA product.
Similar articles
- Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm.
Hogan BM, Hunter MP, Oates AC, Crowhurst MO, Hall NE, Heath JK, Prince VE, Lieschke GJ. Hogan BM, et al. Dev Biol. 2004 Dec 15;276(2):508-22. doi: 10.1016/j.ydbio.2004.09.018. Dev Biol. 2004. PMID: 15581882 - Shh signalling restricts the expression of Gcm2 and controls the position of the developing parathyroids.
Grevellec A, Graham A, Tucker AS. Grevellec A, et al. Dev Biol. 2011 May 15;353(2):194-205. doi: 10.1016/j.ydbio.2011.02.012. Epub 2011 Feb 22. Dev Biol. 2011. PMID: 21349263 - Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid.
Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Xu PX, et al. Development. 2002 Jul;129(13):3033-44. doi: 10.1242/dev.129.13.3033. Development. 2002. PMID: 12070080 Free PMC article. - Impacts of a new transcription factor family: mammalian GCM proteins in health and disease.
Hashemolhosseini S, Wegner M. Hashemolhosseini S, et al. J Cell Biol. 2004 Sep 13;166(6):765-8. doi: 10.1083/jcb.200406097. Epub 2004 Sep 7. J Cell Biol. 2004. PMID: 15353544 Free PMC article. Review. - Chronicles of a switch hunt: gcm genes in development.
Wegner M, Riethmacher D. Wegner M, et al. Trends Genet. 2001 May;17(5):286-90. doi: 10.1016/s0168-9525(01)02275-2. Trends Genet. 2001. PMID: 11335039 Review.
Cited by
- Thymus Inception: Molecular Network in the Early Stages of Thymus Organogenesis.
Figueiredo M, Zilhão R, Neves H. Figueiredo M, et al. Int J Mol Sci. 2020 Aug 11;21(16):5765. doi: 10.3390/ijms21165765. Int J Mol Sci. 2020. PMID: 32796710 Free PMC article. Review. - Identification of a novel gill-specific calpain from rainbow trout (Oncorhynchus mykiss).
Salem M, Rexroad CE, Yao J. Salem M, et al. Fish Physiol Biochem. 2006 Mar;32(1):1-6. doi: 10.1007/s10695-005-0560-2. Fish Physiol Biochem. 2006. PMID: 20035473 - Lineage specification in the fly nervous system and evolutionary implications.
Cattenoz PB, Giangrande A. Cattenoz PB, et al. Cell Cycle. 2013 Sep 1;12(17):2753-9. doi: 10.4161/cc.25918. Epub 2013 Aug 7. Cell Cycle. 2013. PMID: 23966161 Free PMC article. Review. - A role for transcription factor glial cell missing 2 in Ca2+ homeostasis in zebrafish, Danio rerio.
Kumai Y, Kwong RW, Perry SF. Kumai Y, et al. Pflugers Arch. 2015 Apr;467(4):753-65. doi: 10.1007/s00424-014-1544-9. Epub 2014 Jun 5. Pflugers Arch. 2015. PMID: 24893788 - Key separable events in the remodelling of the pharyngeal arches.
Poopalasundaram S, Richardson J, Graham A. Poopalasundaram S, et al. J Anat. 2023 Jul;243(1):100-109. doi: 10.1111/joa.13850. Epub 2023 Feb 23. J Anat. 2023. PMID: 36815518 Free PMC article.
References
- Brown, E. M. (2001) in The Parathyroids: Basic and Clinical Concepts (Academic, San Diego), pp. 167–181.
- Gordon, J., Bennett, A. R., Blackburn, C. C. & Manley, N. R. (2001) Mech. Dev. 103, 141–143. - PubMed
- Gunther, T., Chen, Z. F., Kim, J., Priemel, M., Rueger, J. M., Amling, M., Moseley, J. M., Martin, T. J., Anderson, D. J. & Karsenty, G. (2000) Nature 406, 199–203. - PubMed
- Henrique, D., Adam, J., Myat, A., Chitnis, A., Lewis, J. & Ish-Horowicz, D. (1995) Nature 375, 787–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases