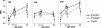Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial - PubMed (original) (raw)
Clinical Trial
Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial
Nina Ekström et al. Infect Immun. 2005 Jan.
Abstract
The licensure of new pneumococcal conjugate vaccines (PCVs) relies on immunogenicity data. When defining correlates of protection, vaccine efficacy data must be included. In the FinOM Vaccine Efficacy Trial, the PncOMPC vaccine showed an efficacy profile similar to that of the licensed PncCRM vaccine despite different antibody responses after primary and booster vaccinations. We determined antibody kinetics and avidities in a subgroup of infants participating in the FinOM trial. A total of 166 infants in three vaccine groups were immunized at 2, 4, 6, and 12 months of age with 7-valent PCV, PncCRM or PncOMPC, or hepatitis B vaccine. Concentrations of serum immunoglobulin G (IgG) against pneumococcal capsular polysaccharides were determined at 2, 4, 6, 7, 12, 13, and 24 months of age, and the avidity index (AI) to serotypes 6B, 19F, and 23F were determined at 7, 12, 13, and 24 months of age by enzyme immunoassay. Both PCVs were highly immunogenic, but they demonstrated different kinetics of antibody response; the concentration of IgG against serotypes 6B, 19F, and 23F declined faster after the third and fourth doses of vaccine in the PncCRM group than in the PncOMPC group. For both PCVs, the mean AI of anti-6B and -23F, but not of anti-19F, increased during the follow-up, which is in line with serotype-specific protection in the FinOM trial. Our data suggest that the kinetics and avidities of antibodies should be considered, in addition to antibody responses, when defining correlates of protection.
Figures
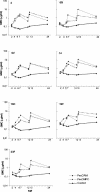
FIG. 1.
GMC against each of the serotypes of a 7-valent pneumococcal conjugate vaccine at different time points in infants immunized at 2, 4, 6, and 12 months of age with PncCRM (n = 54 to 56), PncOMPC (n = 44 to 55), or a control vaccine (n = 53 or 54).
FIG. 2.
Reverse cumulative distribution curves demonstrating the percentages of children achieving various serum IgG antibody concentrations against each of the vaccine serotypes at various ages. The children were immunized at 2, 4, 6, and 12 months of age with a pneumococcal conjugate vaccine, PncCRM (n = 54 to 56) or PncOMPC (n = 44 to 55).
FIG. 3.
Percentages and 95% confidence intervals of infants with an antibody concentrations of ≥0.35 μg/ml at 7, 12, 13, and 24 months of age. The infants were immunized at 2, 4, 6, and 12 months of age with a 7-valent pneumococcal conjugate vaccine, PncCRM (n = 54 to 56) or PncOMPC (n = 44 to 55), or a control vaccine (n = 53 or 54).
FIG. 4.
MAIs and 95% confidence intervals of anti-pneumococcal type 6B, 19F, and 23F capsular polysaccharide IgG antibodies in infants immunized at 2, 4, 6, and 12 months of age with 7-valent pneumococcal conjugate vaccine, PncCRM (n = 53 to 56) or PncOMPC (n = 44 to 53), or a control vaccine (n = 40 to 47).
Similar articles
- Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial.
Ekström N, Väkeväinen M, Verho J, Kilpi T, Käyhty H. Ekström N, et al. Infect Immun. 2007 Apr;75(4):1794-800. doi: 10.1128/IAI.01673-06. Epub 2007 Jan 29. Infect Immun. 2007. PMID: 17261612 Free PMC article. Clinical Trial. - Serum and salivary anti-capsular antibodies in infants and children immunized with the heptavalent pneumococcal conjugate vaccine.
Nurkka A, Ahman H, Korkeila M, Jäntti V, Käyhty H, Eskola J. Nurkka A, et al. Pediatr Infect Dis J. 2001 Jan;20(1):25-33. doi: 10.1097/00006454-200101000-00006. Pediatr Infect Dis J. 2001. PMID: 11176563 - Salivary antibodies induced by the seven-valent PncOMPC conjugate vaccine in the Finnish Otitis Media Vaccine Trial.
Nurkka A, Lahdenkari M, Palmu AA, Käyhty H; FinOM Study Group. Nurkka A, et al. BMC Infect Dis. 2005 May 27;5:41. doi: 10.1186/1471-2334-5-41. BMC Infect Dis. 2005. PMID: 15921511 Free PMC article. Clinical Trial. - Pneumococcal conjugate vaccine for young children.
Selman S, Hayes D, Perin LA, Hayes WS. Selman S, et al. Manag Care. 2000 Sep;9(9):49-52, 54, 56-7 passim. Manag Care. 2000. PMID: 11116663 Review. - Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?
Hausdorff WP, Hoet B, Schuerman L. Hausdorff WP, et al. BMC Pediatr. 2010 Feb 2;10:4. doi: 10.1186/1471-2431-10-4. BMC Pediatr. 2010. PMID: 20122261 Free PMC article. Review.
Cited by
- The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity.
Park DE, Johnson TS, Nonyane BA, Chandir S, Conklin L, Fleming-Dutra KE, Loo JD, Goldblatt D, Whitney CG, O'Brien KL, Deloria Knoll M. Park DE, et al. Pediatr Infect Dis J. 2014 Jan;33 Suppl 2(Suppl 2 Optimum Dosing of Pneumococcal Conjugate Vaccine For Infants 0 A Landscape Analysis of Evidence Supportin g Different Schedules):S130-9. doi: 10.1097/INF.0000000000000081. Pediatr Infect Dis J. 2014. PMID: 24336055 Free PMC article. Review. - Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines.
Poolman J, Frasch C, Nurkka A, Käyhty H, Biemans R, Schuerman L. Poolman J, et al. Clin Vaccine Immunol. 2011 Feb;18(2):327-36. doi: 10.1128/CVI.00402-10. Epub 2010 Dec 1. Clin Vaccine Immunol. 2011. PMID: 21123523 Free PMC article. - Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Carriage Among American Indians.
Grant LR, Hammitt LL, O'Brien SE, Jacobs MR, Donaldson C, Weatherholtz RC, Reid R, Santosham M, O'Brien KL. Grant LR, et al. Pediatr Infect Dis J. 2016 Aug;35(8):907-14. doi: 10.1097/INF.0000000000001207. Pediatr Infect Dis J. 2016. PMID: 27171679 Free PMC article. - Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial.
Ekström N, Väkeväinen M, Verho J, Kilpi T, Käyhty H. Ekström N, et al. Infect Immun. 2007 Apr;75(4):1794-800. doi: 10.1128/IAI.01673-06. Epub 2007 Jan 29. Infect Immun. 2007. PMID: 17261612 Free PMC article. Clinical Trial. - Immunologic screening of children with recurrent otitis media.
Wiertsema SP, Veenhoven RH, Sanders EA, Rijkers GT. Wiertsema SP, et al. Curr Allergy Asthma Rep. 2005 Jul;5(4):302-7. doi: 10.1007/s11882-005-0070-4. Curr Allergy Asthma Rep. 2005. PMID: 15967072 Review.
References
- Åhman, H., H. Käyhty, P. Tamminen, A. Vuorela, F. Malinoski, and J. Eskola. 1996. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr. Infect. Dis. J. 15:134-139. - PubMed
- Anderson, E. L., D. J. Kennedy, K. M. Geldmacher, J. Donnelly, and P. M. Mendelman. 1996. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J. Pediatr. 128:649-653. - PubMed
- Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. - PubMed
- Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical