Liver fibrosis - PubMed (original) (raw)
Review
Liver fibrosis
Ramón Bataller et al. J Clin Invest. 2005 Feb.
Erratum in
- J Clin Invest. 2005 Apr;115(4):1100
Abstract
Liver fibrosis is the excessive accumulation of extracellular matrix proteins including collagen that occurs in most types of chronic liver diseases. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension and often requires liver transplantation. Our knowledge of the cellular and molecular mechanisms of liver fibrosis has greatly advanced. Activated hepatic stellate cells, portal fibroblasts, and myofibroblasts of bone marrow origin have been identified as major collagen-producing cells in the injured liver. These cells are activated by fibrogenic cytokines such as TGF-beta1, angiotensin II, and leptin. Reversibility of advanced liver fibrosis in patients has been recently documented, which has stimulated researchers to develop antifibrotic drugs. Emerging antifibrotic therapies are aimed at inhibiting the accumulation of fibrogenic cells and/or preventing the deposition of extracellular matrix proteins. Although many therapeutic interventions are effective in experimental models of liver fibrosis, their efficacy and safety in humans is unknown. This review summarizes recent progress in the study of the pathogenesis and diagnosis of liver fibrosis and discusses current antifibrotic strategies.
Figures
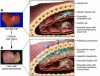
Figure 1
Changes in the hepatic architecture (A) associated with advanced hepatic fibrosis (B). Following chronic liver injury, inflammatory lymphocytes infiltrate the hepatic parenchyma. Some hepatocytes undergo apoptosis, and Kupffer cells activate, releasing fibrogenic mediators. HSCs proliferate and undergo a dramatic phenotypical activation, secreting large amounts of extracellular matrix proteins. Sinusoidal endothelial cells lose their fenestrations, and the tonic contraction of HSCs causes increased resistance to blood flow in the hepatic sinusoid. Figure modified with permission from Science & Medicine (S28).
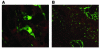
Figure 2
Expression of collagen α1(I) in a model of cholestasis-induced liver fibrosis. Transgenic mice with green fluorescence protein reporter gene under the direction of the collagen α1(I) promoter/enhancers were subjected to bile duct ligation for 2 weeks. (A) Collagen α1(I) was markedly expressed by activated HSCs, but not hepatocytes, in the hepatic parenchyma. Magnification, ×200. (B) Collagen α1(I) is markedly expressed by myofibroblasts around proliferating bile ducts. HSCs proliferate to initiate collagen deposition in the hepatic parenchyma. Magnification, ×40.
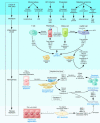
Figure 3
Cellular mechanisms of liver fibrosis. Different types of hepatotoxic agents produce mediators that induce inflammatory actions in hepatic cell types. Damaged hepatocytes and biliary cells release inflammatory cytokines and soluble factors that activate Kupffer cells and stimulate the recruitment of activated T cells. This inflammatory milieu stimulates the activation of resident HSCs into fibrogenic myofibroblasts. Activated HSCs also secrete cytokines that perpetuate their activated state. If the liver injury persists, accumulation of activated HSCs and portal myofibroblasts occurs, synthesizing large amounts of ECM proteins and leading to tissue fibrosis. ECM degradation is inhibited by the actions of cytokines such as TIMPs. Apoptosis of damaged hepatocytes stimulates the fibrogenic actions of HSCs. If the cause of the liver injury is removed, fibrosis is resolved. This phase includes apoptosis of activated HSCs and regeneration of hepatocytes. Collagen is degraded by increased activity of MMPs induced by decreased TIMP expression. CCL21, C-C chemokine ligand 21; MCP-1, monocyte chemoattractant protein–1; MIP-2, macrophage inflammatory protein–2; NS3, HCV nonstructural protein 3; NS5, HCV nonstructural protein 5; PAF, platelet-activating factor.
Figure 4
Reversibility of liver fibrosis in a patient with chronic hepatitis B virus infection after successful treatment with lamivudine. A decrease in smooth muscle actin immunostaining, a marker of fibrogenic myofibroblasts, can be seen in paired liver biopsies before (A) and after (B) therapy. Dark brown granules represent areas stained for smooth muscle actin. Magnification, ×40. Reproduced with permission from Journal of Hepatology (S2).
Similar articles
- Graptopetalum paraguayense Inhibits Liver Fibrosis by Blocking TGF-β Signaling In Vivo and In Vitro.
Hsu WH, Liao SC, Chyan YJ, Huang KW, Hsu SL, Chen YC, Siu ML, Chang CC, Chung YS, Huang CF. Hsu WH, et al. Int J Mol Sci. 2019 May 27;20(10):2592. doi: 10.3390/ijms20102592. Int J Mol Sci. 2019. PMID: 31137784 Free PMC article. - Hepatic stellate cells as a target for the treatment of liver fibrosis.
Bataller R, Brenner DA. Bataller R, et al. Semin Liver Dis. 2001 Aug;21(3):437-51. doi: 10.1055/s-2001-17558. Semin Liver Dis. 2001. PMID: 11586471 Review. - Anti-fibrogenic strategies and the regression of fibrosis.
Kisseleva T, Brenner DA. Kisseleva T, et al. Best Pract Res Clin Gastroenterol. 2011 Apr;25(2):305-17. doi: 10.1016/j.bpg.2011.02.011. Best Pract Res Clin Gastroenterol. 2011. PMID: 21497747 Free PMC article. Review. - Liver fibrosis: Direct antifibrotic agents and targeted therapies.
Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Schuppan D, et al. Matrix Biol. 2018 Aug;68-69:435-451. doi: 10.1016/j.matbio.2018.04.006. Epub 2018 Apr 12. Matrix Biol. 2018. PMID: 29656147 Review. - Relaxin in hepatic fibrosis: What is known and where to head?
Ezhilarasan D. Ezhilarasan D. Biochimie. 2021 Aug;187:144-151. doi: 10.1016/j.biochi.2021.06.001. Epub 2021 Jun 5. Biochimie. 2021. PMID: 34102254 Review.
Cited by
- Telmisartan, an AT1 receptor blocker and a PPAR gamma activator, alleviates liver fibrosis induced experimentally by Schistosoma mansoni infection.
Attia YM, Elalkamy EF, Hammam OA, Mahmoud SS, El-Khatib AS. Attia YM, et al. Parasit Vectors. 2013 Jul 5;6:199. doi: 10.1186/1756-3305-6-199. Parasit Vectors. 2013. PMID: 23829789 Free PMC article. - A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response.
Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, Atkins AR, Barish GD, Gunton JE, Liddle C, Downes M, Evans RM. Ding N, et al. Cell. 2013 Apr 25;153(3):601-13. doi: 10.1016/j.cell.2013.03.028. Cell. 2013. PMID: 23622244 Free PMC article. - Epigenetics in liver disease: from biology to therapeutics.
Hardy T, Mann DA. Hardy T, et al. Gut. 2016 Nov;65(11):1895-1905. doi: 10.1136/gutjnl-2015-311292. Epub 2016 Sep 13. Gut. 2016. PMID: 27624887 Free PMC article. Review. - Hepatic ADC map as an adjunct to conventional abdominal MRI to evaluate hepatic fibrotic and clinical cirrhotic severity in biliary atresia patients.
Peng SS, Jeng YM, Hsu WM, Yang JC, Ho MC. Peng SS, et al. Eur Radiol. 2015 Oct;25(10):2992-3002. doi: 10.1007/s00330-015-3716-1. Epub 2015 Apr 29. Eur Radiol. 2015. PMID: 25921590 - Cluster of differentiation 147 is a key molecule during hepatocellular carcinoma cell-hepatic stellate cell cross-talk in the rat liver.
Ma T, Wang Z, Yang Z, Chen J. Ma T, et al. Mol Med Rep. 2015 Jul;12(1):111-8. doi: 10.3892/mmr.2015.3429. Epub 2015 Mar 4. Mol Med Rep. 2015. PMID: 25738354 Free PMC article.
References
- Friedman SL. Liver fibrosis - from bench to bedside. J. Hepatol. 2003;38(Suppl. 1):S38–S53. - PubMed
- Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N. Engl. J. Med. 2004;350:1646–1654. - PubMed
- Popper H, Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am. J. Med. 1970;49:707–721. - PubMed
- Schaffner F, Klion FM. Chronic hepatitis. Annu. Rev. Med. 1968;19:25–38. - PubMed
- Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin. Liver Dis. 2001;5:315–334, v–vi. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical