Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis - PubMed (original) (raw)
. 2005 Mar 15;102(11):4068-73.
doi: 10.1073/pnas.0500702102. Epub 2005 Mar 7.
Moira K O'Bryan, Stephen Fletcher, Pauline E Crewther, Ulla Aapola, Jeff Craig, Dion K Harrison, Hnin Aung, Nawapen Phutikanit, Robert Lyle, Sarah J Meachem, Stylianos E Antonarakis, David M de Kretser, Mark P Hedger, Pärt Peterson, Bernard J Carroll, Hamish S Scott
Affiliations
- PMID: 15753313
- PMCID: PMC552976
- DOI: 10.1073/pnas.0500702102
Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis
Kylie E Webster et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The production of mature germ cells capable of generating totipotent zygotes is a highly specialized and sexually dimorphic process. The transition from diploid primordial germ cell to haploid spermatozoa requires genome-wide reprogramming of DNA methylation, stage- and testis-specific gene expression, mitotic and meiotic division, and the histone-protamine transition, all requiring unique epigenetic control. Dnmt3L, a DNA methyltransferase regulator, is expressed during gametogenesis, and its deletion results in sterility. We found that during spermatogenesis, Dnmt3L contributes to the acquisition of DNA methylation at paternally imprinted regions, unique nonpericentric heterochromatic sequences, and interspersed repeats, including autonomous transposable elements. We observed retrotransposition of an LTR-ERV1 element in the DNA from Dnmt3L-/- germ cells, presumably as a result of hypomethylation. Later in development, in Dnmt3L-/- meiotic spermatocytes, we detected abnormalities in the status of biochemical markers of heterochromatin, implying aberrant chromatin packaging. Coincidentally, homologous chromosomes fail to align and form synaptonemal complexes, spermatogenesis arrests, and spermatocytes are lost by apoptosis and sloughing. Because Dnmt3L expression is restricted to gonocytes, the presence of defects in later stages reveals a mechanism whereby early genome reprogramming is linked inextricably to changes in chromatin structure required for completion of spermatogenesis.
Figures
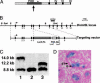
Fig. 1.
Disruption of Dnmt3L and its expression in gonocytes. (A) The Dnmt3L protein, plant homeodomain (PHD)-like domain, nuclear localization signal (*), and C-terminal motifs (I, IV, VI, and IX) relative to the insertion site of the LacZ gene (arrow) (3). (B) Targeted disruption of Dnmt3L.E, _Eco_RI; B, _Bam_HI; PA, polyadenylation signal; PGK-Neo, phosphoglycerate kinase neomycin. (C) Southern blot analysis of _Dra_I-digested ES cell DNA (3′ probe) gave products of 8.8 kb (lane 2), 14.0 kb (lane 1), and 12.2 kb (lane 3) for the WT, targeted, and excised alleles, respectively. (D) LacZ reporter gene expression in gonocytes (G) of newborn testis. ST, seminiferous tubule.
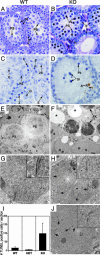
Fig. 2.
_Dnmt3L_–/– germ cells are lost by sloughing and apoptosis after failure of homologous chromosome alignment and synapsis. WT (A, C, E, and G) and _Dnmt3L_–/– (KO) (B, D, F, H, and J) testes are shown. (A) WT d 14 testes. (B) KO d 14 testes containing cells with pyknotic nuclei (Pk) consistent with apoptosis and cells with characteristics of early pachytene spermatocytes (PS). (C) TUNEL staining of d 41 WT. (D) TUNEL staining of d 41 KO reveals an increase in apoptotic cells (A) (brown staining). Germ cell sloughing (Sl) was frequently observed. (E) EM of d 22 WT testes. (F) EM of d 22 KO testes shows many degenerating cells and vacuolation (*) indicative of recent germ cell loss. (G) The tripartite appearance of homologous chromosomes synapsed by means of the SC (Inset) in WT pachytene spermatocytes. (H and J) KO testes contained single unpaired chromosomes and partially paired chromosomes (arrows). Abnormal ribosomal clumps were frequently found (open arrow). (I) Graphical representation of the number of TUNEL-positive cells per section of WT, heterozygous, and KO testes. In, intermediate spermatogonia; E, elongating spermatid; S, Sertoli cell. Spermatocytes: Pl, preleptotene; L, leptotene; Z, zygotene; D, diplotene; 2S, secondary.
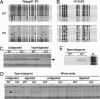
Fig. 3.
Abnormal DNA methylation in _Dnmt3L_–/– germ cells. (A and B) Bisulfite genomic sequencing of H19 and Rasgrf1 paternally (P) imprinted loci from WT and KO germ cells. Each line corresponds to a single strand of DNA, and each circle represents an individual CpG dinucleotide. Filled circles, methylated CpG; open circles, unmethylated CpG; gray circles, incomplete sequence. (C) Portion of a gel showing an AMP-detected DNA methylation polymorphism in KO spermatogonia. Absence of the locus (arrow) only in digested KO DNA indicates loss of methylation. (D) Portion of the gel showing the AMP locus (arrow) detected in KO spermatogonia but not in WT spermatogonia or KO somatic cells. (E) Southern blot confirmation of the AMP locus shown in D. M, size marker.
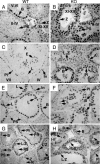
Fig. 4.
Differential detection of histone modifications in _Dnmt3L_–/– germ cells. (A and B) Acetylated H4 was detected in leptotene (L), zygotene (Z), and pachytene (PS) spermatocytes in KO (B) but not in WT (A) testes at all ages (d 21 shown). (C and D) Acetylated H3K9 was detected in leptotene and zygotene spermatocytes in KO (D) but not in WT (C) adult testes (d 42). (E and F) Dimethylated H3K9 was detected in leptotene and zygotene spermatocytes in WT (E) but not in KO (F) testes at all ages (d 21 shown). (G and H) Unmodified histone H3 stained strongly in pachytene spermatocytes of KO (H) but not WT (G) testes. Spermatogonia: In, intermediate; A, type A; B, type B; Pl, preleptotene spermatocyte; R, round spermatid; E, elongating spermatid. Roman numerals indicate seminiferous tubule stage.
Similar articles
- Loss of spermatogonia and wide-spread DNA methylation defects in newborn male mice deficient in DNMT3L.
La Salle S, Oakes CC, Neaga OR, Bourc'his D, Bestor TH, Trasler JM. La Salle S, et al. BMC Dev Biol. 2007 Sep 18;7:104. doi: 10.1186/1471-213X-7-104. BMC Dev Biol. 2007. PMID: 17875220 Free PMC article. - Haploinsufficiency of the paternal-effect gene Dnmt3L results in transient DNA hypomethylation in progenitor cells of the male germline.
Niles KM, Yeh JR, Chan D, Landry M, Nagano MC, Trasler JM. Niles KM, et al. Hum Reprod. 2013 Feb;28(2):519-30. doi: 10.1093/humrep/des395. Epub 2012 Nov 15. Hum Reprod. 2013. PMID: 23159436 Free PMC article. - Meiotic and epigenetic aberrations in Dnmt3L-deficient male germ cells.
Hata K, Kusumi M, Yokomine T, Li E, Sasaki H. Hata K, et al. Mol Reprod Dev. 2006 Jan;73(1):116-22. doi: 10.1002/mrd.20387. Mol Reprod Dev. 2006. PMID: 16211598 - Epigenetic transitions in germ cell development and meiosis.
Kota SK, Feil R. Kota SK, et al. Dev Cell. 2010 Nov 16;19(5):675-86. doi: 10.1016/j.devcel.2010.10.009. Dev Cell. 2010. PMID: 21074718 Review. - Establishment of male-specific epigenetic information.
Rousseaux S, Caron C, Govin J, Lestrat C, Faure AK, Khochbin S. Rousseaux S, et al. Gene. 2005 Jan 31;345(2):139-53. doi: 10.1016/j.gene.2004.12.004. Epub 2005 Jan 25. Gene. 2005. PMID: 15716030 Review.
Cited by
- Sperm epigenetics and male infertility: unraveling the molecular puzzle.
Hosseini M, Khalafiyan A, Zare M, Karimzadeh H, Bahrami B, Hammami B, Kazemi M. Hosseini M, et al. Hum Genomics. 2024 Jun 4;18(1):57. doi: 10.1186/s40246-024-00626-4. Hum Genomics. 2024. PMID: 38835100 Free PMC article. Review. - C19ORF84 connects piRNA and DNA methylation machineries to defend the mammalian germ line.
Zoch A, Konieczny G, Auchynnikava T, Stallmeyer B, Rotte N, Heep M, Berrens RV, Schito M, Kabayama Y, Schöpp T, Kliesch S, Houston B, Nagirnaja L, O'Bryan MK, Aston KI, Conrad DF, Rappsilber J, Allshire RC, Cook AG, Tüttelmann F, O'Carroll D. Zoch A, et al. Mol Cell. 2024 Mar 21;84(6):1021-1035.e11. doi: 10.1016/j.molcel.2024.01.014. Epub 2024 Feb 14. Mol Cell. 2024. PMID: 38359823 - Unlocking the mystery associated with infertility and prostate cancer: an update.
Mukherjee AG, Gopalakrishnan AV. Mukherjee AG, et al. Med Oncol. 2023 Apr 26;40(6):160. doi: 10.1007/s12032-023-02028-3. Med Oncol. 2023. PMID: 37099242 Review. - Genetic manipulation and targeted protein degradation in mammalian systems: practical considerations, tips and tricks for discovery research.
Giandomenico SL, Schuman EM. Giandomenico SL, et al. FEBS Open Bio. 2023 Jul;13(7):1164-1176. doi: 10.1002/2211-5463.13581. Epub 2023 Mar 8. FEBS Open Bio. 2023. PMID: 36815235 Free PMC article. Review. - Epigenetic markers in the embryonal germ cell development and spermatogenesis.
Odroniec A, Olszewska M, Kurpisz M. Odroniec A, et al. Basic Clin Androl. 2023 Feb 23;33(1):6. doi: 10.1186/s12610-022-00179-3. Basic Clin Androl. 2023. PMID: 36814207 Free PMC article. Review.
References
- Li, E. (2002) Nat. Rev. Genet. 3, 662–673. - PubMed
- Surani, M. A. (2001) Nature 414, 122–128. - PubMed
- Aapola, U., Lyle, R., Krohn, K., Antonarakis, S. E. & Peterson, P. (2001) Cytogenet. Cell Genet. 92, 122–126. - PubMed
- Bourc'his, D., Xu, G. L., Lin, C. S., Bollman, B. & Bestor, T. H. (2001) Science 294, 2536–2539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases