Epigenetic alteration at the DLK1-GTL2 imprinted domain in human neoplasia: analysis of neuroblastoma, phaeochromocytoma and Wilms' tumour - PubMed (original) (raw)
Epigenetic alteration at the DLK1-GTL2 imprinted domain in human neoplasia: analysis of neuroblastoma, phaeochromocytoma and Wilms' tumour
D Astuti et al. Br J Cancer. 2005.
Abstract
Epigenetic alterations in the 11p15.5 imprinted gene cluster are frequent in human cancers and are associated with disordered imprinting of insulin-like growth factor (IGF)2 and H19. Recently, an imprinted gene cluster at 14q32 has been defined and includes two closely linked but reciprocally imprinted genes, DLK1 and GTL2, that have similarities to IGF2 and H19, respectively. Both GTL2 and H19 are maternally expressed RNAs with no protein product and display paternal allele promoter region methylation, and DLK1 and IGF2 are both paternally expressed. To determine whether methylation alterations within the 14q32 imprinted domain occur in human tumorigenesis, we investigated the status of the GTL2 promoter differentially methylated region (DMR) in 20 neuroblastoma tumours, 20 phaeochromocytomas and, 40 Wilms' tumours. Hypermethylation of the GTL2 promoter DMR was detected in 25% of neuroblastomas, 10% of phaeochromocytoma and 2.5% of Wilms' tumours. Tumours with GTL2 promoter DMR hypermethylation also demonstrated hypermethylation at an upstream intergenic DMR thought to represent a germline imprinting control element. Analysis of neuroblastoma cell lines revealed that GTL2 DMR hypermethylation was associated with transcriptional repression of GTL2. These epigenetic findings are similar to those reported in Wilms' tumours in which H19 repression and DMR hypermethylation is associated with loss of imprinting (LOI, biallelic expression) of IGF2. However, a neuroblastoma cell line with hypermethylation of the GTL2 promoter and intergenic DMR did not show LOI of DLK1 and although treatment with a demethylating agent restored GTL2 expression and reduced DLK1 expression. As described for IGF2/H19, epigenetic changes at DLK1/GTL2 occur in human cancers. However, these changes are not associated with DLK1 LOI highlighting differences in the imprinting control mechanisms operating in the IGF2-H19 and DLK1-GTL2 domains. GTL2 promoter and intergenic DMR hypermethylation is associated with the loss of GTL2 expression and this may contribute to tumorigenesis in a subset of human cancers.
Figures
Figure 1
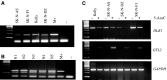
(A) Methylation-specific multiplexed PCR of the DMR upstream GTL2 promoter in neuroblastoma cell lines showing the presence of only methylated allele of 160 bp. M+ is in vitro methylated DNA. (B) Normal blood DNA was used as control showing both unmethylated (120 bp) and methylated (160 bp) allele. (C) RT–PCR analysis of DLK1 and GTL2 in four neuroblastoma cell lines before (−) and after (+) treatment with demethylating agent 5-AzaC. DLK1 expression was reduced, while GTL2 expression was activated following 5-AzaC treatment. Molecular weight marker was a 100-bp ladder (Invitrogen).
Figure 2
Analysis of DLK1 allele expression in the informative SK-N-AS cell line. DLK1 is monoallelically expressed before and following treatment with 5-AzaC.
Figure 3
RT–PCR showing GTL2 expression in neuroblastoma tumours without gain of methylation at 5′ GTL2 DMRs (lanes 1–6) and tumour with gain of methylation at 5′ GTL2 DMRs (lane 7). Low level of GTL2 expression in this tumour may be caused by normal tissue contamination. Lane 8 is a negative control and GAPDH was used as unlinked control gene.
Figure 4
(A) Schematic representation of GTL2 and IG-DMRs in human showing the position of regions analysed: G1=−363 bp → −161 bp upstream, G2=+293 bp → +673 bp downstream, and G3=+1213 bp → +1583 bp downstream of GTL2 transcription start site, GeneBank accession no. AY314975; IG-DMR=nt 51021–51180, GeneBank accession no. AL 117190). (B) Methylation status of the CpGs in the IG-DMR in the normal blood, neuroblastoma cell lines, a Wilms' tumour, two neuroblastoma tumours and a phaeochromocytoma tumour with gain of methylation at the 5′ GTL2 DMRs. (C) Methylation status of GTL2 DMRs in neuroblastoma cell lines. The methylation status is shown in circles. Open and filled circles indicates complete unmethylation and full methylation, respectively. Partially filled circles indicate the degree of partial methylation.
Similar articles
- Imprinting, expression, and localisation of DLK1 in Wilms tumours.
Fukuzawa R, Heathcott RW, Morison IM, Reeve AE. Fukuzawa R, et al. J Clin Pathol. 2005 Feb;58(2):145-50. doi: 10.1136/jcp.2004.021717. J Clin Pathol. 2005. PMID: 15677533 Free PMC article. - Molecular structure of bovine Gtl2 gene and DNA methylation status of Dlk1-Gtl2 imprinted domain in cloned bovines.
Su H, Li D, Hou X, Tan B, Hu J, Zhang C, Dai Y, Li N, Li S. Su H, et al. Anim Reprod Sci. 2011 Aug;127(1-2):23-30. doi: 10.1016/j.anireprosci.2011.07.002. Epub 2011 Jul 19. Anim Reprod Sci. 2011. PMID: 21820255 - Altered expression of imprinted genes in Wilms tumors.
Hubertus J, Lacher M, Rottenkolber M, Müller-Höcker J, Berger M, Stehr M, von Schweinitz D, Kappler R. Hubertus J, et al. Oncol Rep. 2011 Mar;25(3):817-23. doi: 10.3892/or.2010.1113. Epub 2010 Dec 20. Oncol Rep. 2011. PMID: 21174059 - Role of genomic imprinting in Wilms' tumour and overgrowth disorders.
Reeve AE. Reeve AE. Med Pediatr Oncol. 1996 Nov;27(5):470-5. doi: 10.1002/(SICI)1096-911X(199611)27:5<470::AID-MPO14>3.0.CO;2-E. Med Pediatr Oncol. 1996. PMID: 8827076 Review. - Inherited and Sporadic Epimutations at the IGF2-H19 locus in Beckwith-Wiedemann syndrome and Wilms' tumor.
Riccio A, Sparago A, Verde G, De Crescenzo A, Citro V, Cubellis MV, Ferrero GB, Silengo MC, Russo S, Larizza L, Cerrato F. Riccio A, et al. Endocr Dev. 2009;14:1-9. doi: 10.1159/000207461. Epub 2009 Feb 27. Endocr Dev. 2009. PMID: 19293570 Review.
Cited by
- Selective methylation of CpGs at regulatory binding sites controls NNAT expression in Wilms tumors.
Hubertus J, Zitzmann F, Trippel F, Müller-Höcker J, Stehr M, von Schweinitz D, Kappler R. Hubertus J, et al. PLoS One. 2013 Jun 25;8(6):e67605. doi: 10.1371/journal.pone.0067605. Print 2013. PLoS One. 2013. PMID: 23825673 Free PMC article. - Increased expression of angiogenic genes in the brains of mouse meg3-null embryos.
Gordon FE, Nutt CL, Cheunsuchon P, Nakayama Y, Provencher KA, Rice KA, Zhou Y, Zhang X, Klibanski A. Gordon FE, et al. Endocrinology. 2010 Jun;151(6):2443-52. doi: 10.1210/en.2009-1151. Epub 2010 Apr 14. Endocrinology. 2010. PMID: 20392836 Free PMC article. - Uniparentalism in sporadic colorectal cancer is independent of imprint status, and coordinate for chromosomes 14 and 18.
Darbary HK, Dutt SS, Sait SJ, Nowak NJ, Heinaman RE, Stoler DL, Anderson GR. Darbary HK, et al. Cancer Genet Cytogenet. 2009 Mar;189(2):77-86. doi: 10.1016/j.cancergencyto.2008.10.011. Cancer Genet Cytogenet. 2009. PMID: 19215787 Free PMC article. - How Does Reprogramming to Pluripotency Affect Genomic Imprinting?
Perrera V, Martello G. Perrera V, et al. Front Cell Dev Biol. 2019 May 9;7:76. doi: 10.3389/fcell.2019.00076. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31143763 Free PMC article. Review. - Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and -independent pathways.
Lyu Y, Lou J, Yang Y, Feng J, Hao Y, Huang S, Yin L, Xu J, Huang D, Ma B, Zou D, Wang Y, Zhang Y, Zhang B, Chen P, Yu K, Lam EW, Wang X, Liu Q, Yan J, Jin B. Lyu Y, et al. Leukemia. 2017 Dec;31(12):2543-2551. doi: 10.1038/leu.2017.116. Epub 2017 Apr 12. Leukemia. 2017. PMID: 28400619 Free PMC article.
References
- Charlier C, Segers K, Karim L, Shay T, Gyapay G, Cockett N, Georges M (2001) The Callipyge mutation enhances the expression of coregulated imprinted genes in cis without affecting their imprinting status. Nat Genet 27: 367–369 - PubMed
- Cui H, Niemitz EL, Ravenel JD, Onyango P, Brandenburg SA, Lobanenkov VV, Feinberg AP (2001) Loss of imprinting of insulin-like growth factor-II in Wilms' tumor commonly involves altered methylation but not mutations of CTCF or its binding site. Cancer Res 61: 4947–4950 - PubMed
- Feinberg AP, Cui H, Ohlsson R (2002) DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol 12: 389–398 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous