Real-time luminescence reporting of circadian gene expression in mammals - PubMed (original) (raw)
Real-time luminescence reporting of circadian gene expression in mammals
Shin Yamazaki et al. Methods Enzymol. 2005.
Abstract
Luminescence reporters have been used successfully in studies of circadian rhythms. Real-time measurements of circadian variations in gene expression were made in living cells, cultured tissues, and whole organisms. Because this technique is relatively easy and continuous noninvasive measurement from tissue cultures allows for a drastic reduction in the number of experimental animals, we believe this method will become a common technique for studying circadian rhythms. Using a multichannel recording apparatus, it may also become a powerful tool for the discovery of new drugs. In the past, measurements were done using hand-made apparatuses or by modifying commercially available equipment. We, along with other investigators, have developed user-friendly equipment for performing circadian rhythms experiments, and these systems are now available commercially. This article describes the use of luminescence reporters in circadian research and provides detailed methods used in these experiments. One of our goals in this article is to reduce experimental variability in different laboratories by proposing standard protocols.
Figures
Figure 1
Examples of custom-built PMT setup for luminescence recordings. (A) Example of the light-tight box for PMT housing. Four HC135 photon-counting modules are located inside the light-tight box. The box is kept in an environmental chamber in which the temperature is set at 35.5°. A small fan with baffles is used for circulating the air inside the light-tight box and temperature inside of the box stays at 36.5°. (B) Example of the use of a light-tight incubator. Eight HC135 photon-counting modules are located inside the incubator. Black cardboard is used for the shielding of light, and black plastic sheet is placed between the inside glass door and the metal outside door to prevent light leaks.
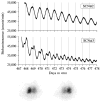
Figure 2
Circadian rhythms of Per1
Similar articles
- Real-time reporting of circadian-regulated gene expression by luciferase imaging in plants and mammalian cells.
Welsh DK, Imaizumi T, Kay SA. Welsh DK, et al. Methods Enzymol. 2005;393:269-88. doi: 10.1016/S0076-6879(05)93011-5. Methods Enzymol. 2005. PMID: 15817294 - Monitoring cell-autonomous circadian clock rhythms of gene expression using luciferase bioluminescence reporters.
Ramanathan C, Khan SK, Kathale ND, Xu H, Liu AC. Ramanathan C, et al. J Vis Exp. 2012 Sep 27;(67):4234. doi: 10.3791/4234. J Vis Exp. 2012. PMID: 23052244 Free PMC article. - Fluorescence/luminescence circadian imaging of complex tissues at single-cell resolution.
Sellix MT, Currie J, Menaker M, Wijnen H. Sellix MT, et al. J Biol Rhythms. 2010 Jun;25(3):228-32. doi: 10.1177/0748730410368016. J Biol Rhythms. 2010. PMID: 20484694 Free PMC article. - The systemic control of circadian gene expression.
Gerber A, Saini C, Curie T, Emmenegger Y, Rando G, Gosselin P, Gotic I, Gos P, Franken P, Schibler U. Gerber A, et al. Diabetes Obes Metab. 2015 Sep;17 Suppl 1:23-32. doi: 10.1111/dom.12512. Diabetes Obes Metab. 2015. PMID: 26332965 Review. - Circadian rhythms lit up in Chlamydomonas.
Breton G, Kay SA. Breton G, et al. Genome Biol. 2006;7(4):215. doi: 10.1186/gb-2006-7-4-215. Epub 2006 Apr 28. Genome Biol. 2006. PMID: 16677428 Free PMC article. Review.
Cited by
- Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2::LUC bioluminescence.
Leise TL, Wang CW, Gitis PJ, Welsh DK. Leise TL, et al. PLoS One. 2012;7(3):e33334. doi: 10.1371/journal.pone.0033334. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479387 Free PMC article. - Tissue-specific function of Period3 in circadian rhythmicity.
Pendergast JS, Niswender KD, Yamazaki S. Pendergast JS, et al. PLoS One. 2012;7(1):e30254. doi: 10.1371/journal.pone.0030254. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22253927 Free PMC article. - Circadian clocks in rat skin and dermal fibroblasts: differential effects of aging, temperature and melatonin.
Sandu C, Liu T, Malan A, Challet E, Pévet P, Felder-Schmittbuhl MP. Sandu C, et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48. doi: 10.1007/s00018-014-1809-7. Epub 2015 Jan 7. Cell Mol Life Sci. 2015. PMID: 25563487 Free PMC article. - Interaction of MAGED1 with nuclear receptors affects circadian clock function.
Wang X, Tang J, Xing L, Shi G, Ruan H, Gu X, Liu Z, Wu X, Gao X, Xu Y. Wang X, et al. EMBO J. 2010 Apr 21;29(8):1389-400. doi: 10.1038/emboj.2010.34. Epub 2010 Mar 18. EMBO J. 2010. PMID: 20300063 Free PMC article. - Slice preparation, organotypic tissue culturing and luciferase recording of clock gene activity in the suprachiasmatic nucleus.
Savelyev SA, Larsson KC, Johansson AS, Lundkvist GB. Savelyev SA, et al. J Vis Exp. 2011 Feb 15;(48):2439. doi: 10.3791/2439. J Vis Exp. 2011. PMID: 21372784 Free PMC article.
References
- Asai M, Yamaguchi S, Isejima H, Jonouchi M, Moriya T, Shibata S, Kobayashi M, Okamura H. Visualization of mPer1 transcription in vitro: NMDA induces a rapid phase shift of mPer1 gene in cultured SCN. Curr Biol. 2001;11:1524–1527. - PubMed
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. - PubMed
- Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. - PubMed
- Brandes C, Plautz JD, Stanewsky R, Jamison CF, Straume M, Wood KV, Kay SA, Hall JC. Novel features of drosophila period Transcription revealed by real-time luciferase reporting. Neuron. 1996;16:687–692. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous