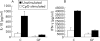Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis - PubMed (original) (raw)
Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis
U G Strauch et al. Gut. 2005 Nov.
Abstract
Background: The resident flora plays a critical role in initiation and perpetuation of intestinal inflammation, as demonstrated in experimental models of colitis where animals fail to develop disease under germ free conditions. However, the importance of exposure to commensal bacteria before the onset of colitis is unclear. Our aim was to investigate the influence of previous exposure of donor animals to bacterial antigens on colitis development using a transfer model.
Methods: Clinical course and histology were evaluated after transfer of CD4(+)CD62L(+) lymphocytes from germ free and conventionally housed donor mice into SCID recipients. Cotransfer of CD4(+)CD62L(+) cells with CD4(+)CD62L(- )lymphocytes from both groups of mice was initiated. Lymphocytes were analysed by FACS, polarisation potential of cells determined, and cytokines measured within the supernatant by enzyme linked immunosorbent assay.
Results: Animals that received cells from germ free donors developed an earlier onset of colitis compared with mice reconstituted with lymphocytes from conventionally housed animals. Additionally, CD4(+)CD62L(- )cells from germ free mice were not able to abrogate colitis induced by cotransfer with CD4(+)CD62L(+) lymphocytes whereas CD4(+)CD62L(- )T cells from normal mice ameliorated disease. The higher percentage of CD4(+)GITR(+) expressing lymphocytes and the production of interleukin 10 after priming by dendritic cells suggests the presence of T(reg) cells within the CD4(+)CD62L(+) lymphocyte subset derived from conventional housed mice and assumes a lack of T(reg) cells within germ free mice.
Conclusion: The results indicate that bacterial antigens are crucial for the generation and/or expansion of T(reg) cells in a healthy individual. Therefore, bacterial colonisation is of great importance in maintaining the immunological balance.
Figures
Figure 1
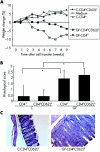
Earlier onset of colitis after transfer of CD4+ and CD4+CD62L+ T cells derived from germ free (GF) animals compared with conventionally (C) housed mice into SCID animals. (A) Changes in weight were monitored over time and are shown as weekly percentage of changes in weight from baseline. (B) Histological score was determined at the end of week 9, as described in the methods section. Values are mean (SD). *p<0.05 versus mice that received cells from conventional donors. (C) Representative colonic haematoxylin-eosin sections from both animal groups are shown (magnification 100×). Data are representative of three independent experiments, n = 5 mice per group.
Figure 2
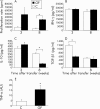
Comparison of cytokine production and proliferation of mesenteric lymph node (MLN) cells from animals reconstituted with cells derived from germ free (GF) or conventionally (C) housed mice. (A) CD4+CD62L+ cells from GF and C donor mice were transferred into SCID recipients and MLN from 3–4 animals from each group were harvested three and eight weeks after transfer. MLN cells were isolated from pooled organs and incubated for 48 hours. Thymidine was added for the last 16 hours of the incubation time and proliferation was measured. (B–D) Additionally, pooled MLN cells from 3–4 animals in each group derived from animals three and eight weeks after transfer were incubated in quadruplicate cultures for 24 hours in the presence of plate bound anti-CD3. Cytokine concentrations of interferon γ (IFN-γ) (B), interleukin 10 (IL-10) (C), and transforming growth factor β1 (TGF-β1) (D) were measured in supernatants by enzyme linked immunosorbent assay. (E) Total RNA was isolated from individual colonic tissue at the end of the experiment (n = 5 mice per group), transcribed, and specific mRNA of the proinflammatory cytokine tumour necrosis factor α (TNF-α) was quantified using a Light cycler.
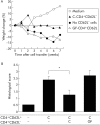
Figure 3
CD4+CD62L− T lymphocytes from germ free (GF) animals were unable to protect from colitis. Equal numbers of CD4+CD62L− T cells from GF or conventional (C) donor mice were cotransferred with CD4+CD62L+ lymphocytes derived from conventionally housed animals into SCID recipients. (A) Weight changes were monitored over time and are shown as percentage of weekly changes compared with baseline. Labelling indicates the additional cotransferred lymphocyte population. (B) Histological scores were determined at the end of week 7, as described in the methods section. Data are representative of three independent experiments, n = 5 mice per group. Values are mean (SD). *p<0.05, significantly different from mice that received only cells from conventionally housed donors without cotransferred lymphocytes.
Figure 4
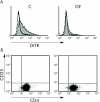
FACS analysis of CD4+CD62L+ lymphocytes from germ free (GF) and conventionally (C) housed mice. CD4+CD62L+ T cells were isolated, and FACS analysis for expression of glucocorticoid induced tumour necrosis factor receptor related protein (GITR) and CD25 was performed. (A) GITR staining of lymphocytes from C housed mice and GF animals. The isotype control is indicated as the unfilled line. (B) Lack of CD25 expression by CD4+CD62L+ T cells derived from both groups of mice. A representative of four separate analyses is shown.
Figure 5
FoxP3 expression and functional analysis of CD4+CD62L+ and CD4+CD62L− lymphocytes from germ free (GF) and conventionally (C) housed mice. (A) CD4+CD62L+ and CD4+CD62L− lymphocytes from C and GF housed animals were isolated and reverse transcription-polymerase chain reaction analyses were conducted for expression of Foxp3 and β-actin. Data are representative of three independent experiments. (B, C) Additionally, CD4+CD62L+ and CD4+CD62L− T cells from GF or C donor mice were incubated in quadruplicate cultures for 24 hours on plate bound anti-CD3 and cytokine concentrations of interleukin 10 (IL-10) and interferon γ (IFN-γ) were measured in supernatants by enzyme linked immunosorbent assay. Data are representative of four independent experiments. Values are mean (SEM). *p<0.05, significant difference between data received from T lymphocytes derived from GF mice compared with C animals.
Figure 6
CD4+CD62L+ T cells from conventionally (C) housed mice secrete higher levels of interleukin 10 (IL-10) after coculture with stimulated bone marrow derived dendritic cells (BM-DC) than lymphocytes from germ free (GF) animals. CD4+CD62L+ T cells were isolated from both animal groups and cultured with unstimulated or CpG stimulated BM-DC for seven days, followed by restimulation of T lymphocytes in quadruplicate cultures with plate bound anti-CD3 and soluble anti-CD28 for 48 hours. Interleukin 10 (IL-10) (A) and interferon γ (IFN-γ) (B) levels were measured in the supernatant of T cells by enzyme linked immunosorbent assay. Data are representative of three independent experiments. Values are mean (SEM). *p<0.05, significant difference between data received from T lymphocytes derived from GF mice compared with C animals.
Similar articles
- T cell-dependent protective effects of CpG motifs of bacterial DNA in experimental colitis are mediated by CD11c+ dendritic cells.
Hofmann C, Dunger N, Grunwald N, Hämmerling GJ, Hoffmann P, Schölmerich J, Falk W, Obermeier F. Hofmann C, et al. Gut. 2010 Oct;59(10):1347-54. doi: 10.1136/gut.2009.193177. Epub 2010 Aug 23. Gut. 2010. PMID: 20732920 - CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction.
Bleich A, Janus LM, Smoczek A, Westendorf AM, Strauch U, Mähler M, Hedrich HJ, Fichtner-Feigl S, Schölmerich J, Falk W, Hofmann C, Obermeier F. Bleich A, et al. Gastroenterology. 2009 Jan;136(1):278-87. doi: 10.1053/j.gastro.2008.09.022. Epub 2008 Sep 25. Gastroenterology. 2009. PMID: 18952084 - Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis.
Nemoto Y, Kanai T, Kameyama K, Shinohara T, Sakamoto N, Totsuka T, Okamoto R, Tsuchiya K, Nakamura T, Sudo T, Matsumoto S, Watanabe M. Nemoto Y, et al. J Immunol. 2009 Oct 15;183(8):5059-68. doi: 10.4049/jimmunol.0803684. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786550 - Control of immune pathology by regulatory T cells.
Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Powrie F, et al. Novartis Found Symp. 2003;252:92-8; discussion 98-105, 106-14. Novartis Found Symp. 2003. PMID: 14609214 Review. - Immuno-bacterial homeostasis in the gut: new insights into an old enigma.
Elson CO, Cong Y, Iqbal N, Weaver CT. Elson CO, et al. Semin Immunol. 2001 Jun;13(3):187-94. doi: 10.1006/smim.2001.0312. Semin Immunol. 2001. PMID: 11394961 Review.
Cited by
- Induced and natural regulatory T cells in the development of inflammatory bowel disease.
Mayne CG, Williams CB. Mayne CG, et al. Inflamm Bowel Dis. 2013 Jul;19(8):1772-88. doi: 10.1097/MIB.0b013e318281f5a3. Inflamm Bowel Dis. 2013. PMID: 23656897 Free PMC article. Review. - The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease.
Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. Himmel ME, et al. Immunology. 2008 Oct;125(2):145-53. doi: 10.1111/j.1365-2567.2008.02939.x. Immunology. 2008. PMID: 18798918 Free PMC article. Review. - Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic?
Weiss ST. Weiss ST. J Allergy Clin Immunol. 2011 May;127(5):1128-30. doi: 10.1016/j.jaci.2011.02.025. Epub 2011 Mar 16. J Allergy Clin Immunol. 2011. PMID: 21411129 Free PMC article. No abstract available. - Beta-lactam antibiotics modulate T-cell functions and gene expression via covalent binding to cellular albumin.
Mor F, Cohen IR. Mor F, et al. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2981-6. doi: 10.1073/pnas.1215722110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382225 Free PMC article. - B Lymphocyte Development in the Bursa of Fabricius of Young Broilers is Influenced by the Gut Microbiota.
Cheng J, Lei H, Xie C, Chen J, Yi X, Zhao F, Yuan Y, Chen P, He J, Luo C, Shu D, Qu H, Ji J. Cheng J, et al. Microbiol Spectr. 2023 Mar 14;11(2):e0479922. doi: 10.1128/spectrum.04799-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36917000 Free PMC article.
References
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 2003;3:521–33. - PubMed
- Elson CO, Cong Y, Brandwein S, et al. Experimental models to study molecular mechanisms underlying intestinal inflammation. Ann N Y Acad Sci 1998;859:85–95. - PubMed
- Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol 2000;67:267–78. - PubMed
- Elson CO, Cong Y, Iqbal N, et al. Immuno-bacterial homeostasis in the gut: new insights into an old enigma. Semin Immunol 2001;13:187–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials