Out-of-plane motions in open sliding clamps: molecular dynamics simulations of eukaryotic and archaeal proliferating cell nuclear antigen - PubMed (original) (raw)
Out-of-plane motions in open sliding clamps: molecular dynamics simulations of eukaryotic and archaeal proliferating cell nuclear antigen
Steven L Kazmirski et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Sliding clamps are ring-like multimeric proteins that encircle duplex DNA and serve as mobile DNA-bound platforms that are essential for efficient DNA replication and repair. Sliding clamps are placed on DNA by clamp loader complexes, in which the clamp-interacting elements are organized in a right-handed spiral assembly. To understand how the flat, ring-like clamps might interact with the spiral interaction surface of the clamp loader complex, we have performed molecular dynamics simulations of sliding clamps (proliferating cell nuclear antigen from the budding yeast, humans, and an archaeal species) in which we have removed one of the three subunits so as to release the constraint of ring closure. The simulations reveal significant structural fluctuations corresponding to lateral opening and out-of-plane distortions of the clamp, which result principally from bending and twisting of the beta-sheets that span the intermolecular interfaces, with smaller but similar contributions from beta-sheets that span the intramolecular interfaces within each subunit. With the integrity of these beta-sheets intact, the predominant fluctuations seen in the simulations are oscillations between lateral openings and right-handed spirals. The tendency for clamps to adopt a right-handed spiral conformation implies that once opened, the conformation of the clamp can easily match the spiraling of clamp loader subunits, a feature that is intrinsic to the recognition of DNA and subsequent hydrolysis of ATP by the clamp-bound clamp loader complex.
Figures
Fig. 1.
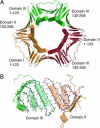
Overview of PCNA architecture. (A) Crystal structure of PCNA from S. cerevisiae (PDB ID code 1PLQ) (8). The structure consists of three subunits, each with two domains connected by a long linker. In the molecular dynamics simulation, one subunit was removed. The domains in the simulated structure are referred to as domains I, II, III and IV. (B) A view of the β-sheet at the intermolecular interface. The hydrogen bonds between the interfacial strands, indicated as dotted lines, are maintained during the simulations even when the structure forms a large, right-handed spiral (see Fig. 6).
Fig. 2.
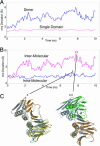
Comparisons between simulated structures and the starting structure reveal rigid body motions between domains. (A) rms deviation in Cα positions from the starting structure for the yeast I simulation. The large deviations over the entire dimer structure (blue) are a result of rigid body motions between the individual domains. The rms deviation in Cα atom positions for domain I of yeast I (magenta) is generally <≈1.5 Å, suggesting that the structures are stable. (B) The rms deviation in the Cα positions for domain I compared with the starting structure (blue) when domain II is matched onto the starting structure. The average deviation is ≈3 Å, suggesting a relatively small rigid body motion of domain II with respect to domain I. (C) A comparison to the crystal structure (gray) is shown for the structure at 7.3 ns. A larger deviation is observed at the intermolecular interface when measuring the rms deviation in Cα positions for domain III (magenta) when domain II is matched onto the starting structure. These larger deviations result from the deformation of the β-sheet at the intermolecular interface and allow for a large displacement of domain III, as shown for the structure at 7.8 ns. The graphs were window-averaged over a 50-ps window.
Fig. 3.
Lateral and out-of-plane movements for PCNA simulations. (A) A trimer was constructed artificially from the simulated dimers, and the shortest distances between any atoms in domain I and an artificial domain VI (see Fig. 1) were calculated. (B) Using just the simulated dimer, domain IV was matched to the crystal structure, and the displacement perpendicular to the ring formed by the starting structure was calculated for domain I. For seven of the nine simulations, the displacement was mainly in a positive direction, corresponding to a right-hand spiral (see Fig. 8_B_). For clarity, the instantaneous displacements were smoothed by averaging over 50 ps.
Fig. 4.
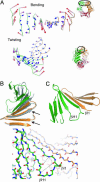
β-sheet distortions underlying the primary domain displacements of simulated PCNA. (A) The two lowest-frequency normal modes are displayed for the intermolecular β-sheet in the yeast I simulation. The lowest-frequency mode involves a bending of the β-sheet; the next lowest-frequency mode shows a twisting of the β-sheet. For each mode, the displacement of each Cα atom from the mean position is indicated by an arrow, the length of which is scaled by an arbitrary scale factor for clarity. Larger displacements are colored red, and smaller ones are colored blue. (B) The intermolecular β-sheet from the yeast crystal structure is shown in gray. When the molecular dynamics simulation at 7.8 ns is matched onto strands of the crystal structure indicated by arrows, a significant right-handed twist to the sheet can be seen. A significant spiraling is observed at 7.8 ns, suggesting that the twisting of the β-sheet is responsible. (C) In yeast III, a left-handed spiral was observed. This conformation is correlated with the tearing of the β-sheet in domain I (hydrogen bonds between residues 105 and 110 and residues 103 and 112 break, indicated by dotted lines in the stick representation of the backbone).
Fig. 5.
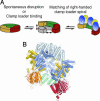
Schematic shows how PCNA may open. In solution, PCNA is normally in a closed-ring form. Either spontaneously or through its initial interaction with RFC, the clamp opens. When domain VI of the simulation is superimposed on the corresponding domain in the RFC–PCNA crystal structure, the right-handed spiraling of the clamp follows the spiral of RFC and would allow for contact between PCNA and all five clamp loader subunits.
Comment in
- The opened processivity clamp slides into view.
Jeruzalmi D. Jeruzalmi D. Proc Natl Acad Sci U S A. 2005 Oct 18;102(42):14939-40. doi: 10.1073/pnas.0507120102. Epub 2005 Oct 10. Proc Natl Acad Sci U S A. 2005. PMID: 16217036 Free PMC article. No abstract available.
Similar articles
- The mechanical properties of PCNA: implications for the loading and function of a DNA sliding clamp.
Adelman JL, Chodera JD, Kuo IF, Miller TF 3rd, Barsky D. Adelman JL, et al. Biophys J. 2010 Jun 16;98(12):3062-9. doi: 10.1016/j.bpj.2010.03.056. Biophys J. 2010. PMID: 20550919 Free PMC article. - Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex.
Bowman GD, O'Donnell M, Kuriyan J. Bowman GD, et al. Nature. 2004 Jun 17;429(6993):724-30. doi: 10.1038/nature02585. Nature. 2004. PMID: 15201901 - Recognition of a Key Anchor Residue by a Conserved Hydrophobic Pocket Ensures Subunit Interface Integrity in DNA Clamps.
Perumal SK, Xu X, Yan C, Ivanov I, Benkovic SJ. Perumal SK, et al. J Mol Biol. 2019 Jun 28;431(14):2493-2510. doi: 10.1016/j.jmb.2019.04.035. Epub 2019 Apr 30. J Mol Biol. 2019. PMID: 31051173 Free PMC article. - Cellular DNA replicases: components and dynamics at the replication fork.
Johnson A, O'Donnell M. Johnson A, et al. Annu Rev Biochem. 2005;74:283-315. doi: 10.1146/annurev.biochem.73.011303.073859. Annu Rev Biochem. 2005. PMID: 15952889 Review. - The RFC clamp loader: structure and function.
Yao NY, O'Donnell M. Yao NY, et al. Subcell Biochem. 2012;62:259-79. doi: 10.1007/978-94-007-4572-8_14. Subcell Biochem. 2012. PMID: 22918590 Free PMC article. Review.
Cited by
- Segmented transition pathway of the signaling protein nitrogen regulatory protein C.
Lei M, Velos J, Gardino A, Kivenson A, Karplus M, Kern D. Lei M, et al. J Mol Biol. 2009 Sep 25;392(3):823-36. doi: 10.1016/j.jmb.2009.06.065. Epub 2009 Jul 1. J Mol Biol. 2009. PMID: 19576227 Free PMC article. - Analysis of the role of PCNA-DNA contacts during clamp loading.
McNally R, Bowman GD, Goedken ER, O'Donnell M, Kuriyan J. McNally R, et al. BMC Struct Biol. 2010 Jan 30;10:3. doi: 10.1186/1472-6807-10-3. BMC Struct Biol. 2010. PMID: 20113510 Free PMC article. - Open clamp structure in the clamp-loading complex visualized by electron microscopic image analysis.
Miyata T, Suzuki H, Oyama T, Mayanagi K, Ishino Y, Morikawa K. Miyata T, et al. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13795-800. doi: 10.1073/pnas.0506447102. Epub 2005 Sep 16. Proc Natl Acad Sci U S A. 2005. PMID: 16169902 Free PMC article. - The mechanical properties of PCNA: implications for the loading and function of a DNA sliding clamp.
Adelman JL, Chodera JD, Kuo IF, Miller TF 3rd, Barsky D. Adelman JL, et al. Biophys J. 2010 Jun 16;98(12):3062-9. doi: 10.1016/j.bpj.2010.03.056. Biophys J. 2010. PMID: 20550919 Free PMC article. - Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity.
Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, Groves JT, Kuriyan J. Stratton M, et al. Elife. 2014 Jan 28;3:e01610. doi: 10.7554/eLife.01610. Elife. 2014. PMID: 24473075 Free PMC article.
References
- Kong, X. P., Onrust, R., O'Donnell, M. & Kuriyan, J. (1992) Cell 69, 425–437. - PubMed
- Stukenberg, P. T., Studwell-Vaughan, P. S. & O'Donnell, M. (1991) J. Biol. Chem. 266, 11328–11334. - PubMed
- Stillman, B. (1994) Cell 78, 725–728. - PubMed
- Kelman, Z. & O'Donnell, M. (1995) Annu. Rev. Biochem. 64, 171–200. - PubMed
- O'Donnell, M. (1992) BioEssays 14, 105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM038839/GM/NIGMS NIH HHS/United States
- F32 GM066586/GM/NIGMS NIH HHS/United States
- GM45547/GM/NIGMS NIH HHS/United States
- F32 GM066586-01/GM/NIGMS NIH HHS/United States
- F32 GM066586-02/GM/NIGMS NIH HHS/United States
- GM38839/GM/NIGMS NIH HHS/United States
- R01 GM045547/GM/NIGMS NIH HHS/United States
- R01 GM038839/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases