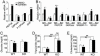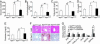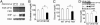Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice - PubMed (original) (raw)
Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice
Yanqiao Zhang et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Farnesoid X receptor (FXR) plays an important role in maintaining bile acid and cholesterol homeostasis. Here we demonstrate that FXR also regulates glucose metabolism. Activation of FXR by the synthetic agonist GW4064 or hepatic overexpression of constitutively active FXR by adenovirus-mediated gene transfer significantly lowered blood glucose levels in both diabetic db/db and wild-type mice. Consistent with these data, FXR null mice exhibited glucose intolerance and insulin insensitivity. We further demonstrate that activation of FXR in db/db mice repressed hepatic gluconeogenic genes and increased hepatic glycogen synthesis and glycogen content by a mechanism that involves enhanced insulin sensitivity. In view of its central roles in coordinating regulation of both glucose and lipid metabolism, we propose that FXR agonists are promising therapeutic agents for treatment of diabetes mellitus.
Figures
Fig. 1.
The FXR agonist GW4064 regulates glucose and lipid homeostasis in an FXR-dependent manner. (A) Murine primary hepatocytes were treated with either vehicle (DMSO) or GW4064 (1 μM) for 24 h. mRNA levels were quantified by real-time PCR and normalized to cyclophilin. F1, 6Pase, fructose 1,6-bis phosphatase. (B_–_E) Wild-type and FXR–/– mice were gavaged with either vehicle (open bars) or GW4064 (filled bars) for 11 days (n = 6 per group). After a 6-h fast, livers were removed and hepatic mRNA levels were quantified by real-time PCR (B). Plasma glucose (C), triglyceride (D), and cholesterol (E) levels were determined after a 6-h fast. *, P < 0.05. **, P < 0.01 versus control (A and C_–_E) or vehicle-treated wild-type group (B), unless specifically indicated.
Fig. 2.
GW4064 treatment of db/db mice results in hypoglycemia and hypolipidemia. (A_–_F) Eighteen-week-old female leprdb/db mice (n = 6 for vehicle-treated group, n = 5 for GW4064-treated group) and their lean littermates Leprdb/? (n = 4) were treated daily with either vehicle (open bars) or GW4064 (filled bars) for 5 days. After a 16-h fast, plasma levels of glucose (A), β-hydroxybutyrate (B), triglyceride (C), free fatty acids (FFA) (D), and total cholesterol (E) were determined. (F) Liver sections were stained with hematoxylin/eosin (H&E) (Upper) or oil red O (Lower). (Magnification: ×400.) (G) Determination of hepatic mRNA levels by real-time PCR. Glut2, glucose transporter 2. *, P < 0.05. **, P < 0.01 versus leprdb/? mice unless specifically indicated.
Fig. 3.
GW4064 treatment enhances insulin signaling and glycogen storage in the livers of db/db mice. Mice were treated with GW4064 for 5 days (A, B, D, and E). (A and B) Hepatic glycogen levels were determined by periodic acid-Schiff staining (A) or colorimetric assay (B). (Magnification: A, ×200.) (C) Murine primary hepatocytes were treated in triplicate with DMSO or GW4064 (1 μM) for 24 h, followed by incubation with media containing 14C-
d
-glucose for 2 h. The incorporation of 14C-
d
-glucose into glycogen was quantified. (D) Determination of phosphorylation levels of hepatic Akt and GSK3β in db/db mice. (E) Determination of phosphotyrosine (pY) levels of hepatic IRS-1 or IRS-2 in db/db mice. *, P < 0.05. **, P < 0.01 versus vehicle-treated primary hepatocytes or leprdb/? mice unless specifically indicated.
Fig. 4.
Hepatic expression of FXR-VP16 decreases plasma glucose and cholesterol levels in nondiabetic mice. (A_–_C) C57BL/6J mice were separately transfused with Ad-VP16 (VP16) or Ad-FXR-VP16 (FXR) or saline alone (WT) by tail vain injection (n = 8 per group). After 7 days, mice were fasted for 6 h before being killed. Hepatic mRNA levels were quantified by Northern blot assay (A), and plasma cholesterol (B) and glucose (C) levels were determined. (D) Hepatic expression of FXRα2-VP16 lowers plasma glucose levels in FXR–/– mice. FXR–/– mice were either separately transfused with the indicated adenovirus or saline alone (KO) by tail vain injection (n = 6 per group). After 7 days, mice were fasted for 16 h before being killed. Hepatic mRNA levels were determined by Northern blot assay (Inset), and plasma glucose levels were determined. *, P < 0.05. **, P < 0.01. #, P < 0.001 versus mice transfused with Ad-VP16.
Fig. 5.
Hepatic expression of FXR-VP16 significantly improves hyperglycemia in db/db mice. (A) Nine-week-old female db/db mice were transfused with adenovirus expressing VP16 alone (open bars) or FXRα2-VP16 (filled bars) by tail vain injection (n = 6 per group). Plasma glucose levels were determined after a 6-h fast on the indicated day after infection. (B) Hepatic mRNA levels were determined by Northern blot assay (Inset) or real-time PCR in db/db mice on day 13 after adenoviral injection. (C) Plasma insulin levels in db/db mice transfused with the indicated adenovirus were measured on day 13 after a 6-h fast. (D) Glucose tolerance test (GTT). db/db mice were transfused with the indicated adenovirus and, on day 7, they were fasted for 16 h before a GTT (n = 6 per group). (E) Determination of phosphotyrosine (pY) levels of hepatic IRS-1 and IRS-2. (F) Determination of hepatic phosphorylated GSK3β and glycogen levels. *, P < 0.05 and **, P < 0.01 versus db/db mice transfused with VP16.
Fig. 6.
A central role for FXR in glucose and lipid homeostasis. (A and B) Impaired glucose tolerance and insulin sensitivity in FXR–/– mice. A glucose tolerance test was performed after a 16-h fast (A) and insulin tolerance test (0.85 unit/kg insulin) was performed after a 5-h fast (B) in wild-type and FXR–/– mice (n = 7 per group). (C) Impaired insulin sensitivity in the liver of FXR–/– mice. Overnight-fasted wild-type and FXR–/– mice were given a bolus injection of 5 units/kg insulin or saline alone (n = 2 per group). After 5 min, the liver was removed and hepatic phosphotyrosine (pY) levels of IRS-2 were determined after immunoprecipitation. (D) Coordinate regulation of glucose and lipid metabolism by FXR in db/db mice. Activation of FXR represses hepatic gluconeogenesis and increases hepatic glycogen storage by sensitizing insulin action, resulting in decreased plasma glucose levels. Activation of FXR also lowers plasma triglyceride levels by repressing triglyceride synthesis (via repressing SREBP-1c) and increasing triglyceride clearance from blood. In addition, activation of FXR lowers plasma cholesterol levels, possibly as a result of increased SR-BI and lethithin:cholesterol acetyltransferase (LCAT) expression. *, P < 0.05; **, P < 0.01.
Similar articles
- The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice.
Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. Cariou B, et al. J Biol Chem. 2006 Apr 21;281(16):11039-49. doi: 10.1074/jbc.M510258200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446356 - Regulation of carbohydrate metabolism by the farnesoid X receptor.
Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF, Burris TP. Stayrook KR, et al. Endocrinology. 2005 Mar;146(3):984-91. doi: 10.1210/en.2004-0965. Epub 2004 Nov 24. Endocrinology. 2005. PMID: 15564327 - The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition.
Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC, Kuipers F, Staels B. Duran-Sandoval D, et al. J Biol Chem. 2005 Aug 19;280(33):29971-9. doi: 10.1074/jbc.M501931200. Epub 2005 May 16. J Biol Chem. 2005. PMID: 15899888 - Role of bile acids and bile acid receptors in metabolic regulation.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Lefebvre P, et al. Physiol Rev. 2009 Jan;89(1):147-91. doi: 10.1152/physrev.00010.2008. Physiol Rev. 2009. PMID: 19126757 Review. - FXR an emerging therapeutic target for the treatment of atherosclerosis.
Mencarelli A, Fiorucci S. Mencarelli A, et al. J Cell Mol Med. 2010 Jan;14(1-2):79-92. doi: 10.1111/j.1582-4934.2009.00997.x. J Cell Mol Med. 2010. PMID: 20041971 Free PMC article. Review.
Cited by
- Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review.
He FF, Li YM. He FF, et al. J Ovarian Res. 2020 Jun 17;13(1):73. doi: 10.1186/s13048-020-00670-3. J Ovarian Res. 2020. PMID: 32552864 Free PMC article. Review. - Bile Acids as Hormones: The FXR-FGF15/19 Pathway.
Kliewer SA, Mangelsdorf DJ. Kliewer SA, et al. Dig Dis. 2015;33(3):327-31. doi: 10.1159/000371670. Epub 2015 May 27. Dig Dis. 2015. PMID: 26045265 Free PMC article. Review. - Maternal glucose homeostasis is impaired in mouse models of gestational cholestasis.
Bellafante E, McIlvride S, Nikolova V, Fan HM, Manna LB, Chambers J, Machirori M, Banerjee A, Murphy K, Martineau M, Schoonjans K, Marschall HU, Jones P, Williamson C. Bellafante E, et al. Sci Rep. 2020 Jul 13;10(1):11523. doi: 10.1038/s41598-020-67968-6. Sci Rep. 2020. PMID: 32661285 Free PMC article. - A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor.
Li G, Lin W, Araya JJ, Chen T, Timmermann BN, Guo GL. Li G, et al. Toxicol Appl Pharmacol. 2012 Jan 15;258(2):268-74. doi: 10.1016/j.taap.2011.11.006. Epub 2011 Dec 4. Toxicol Appl Pharmacol. 2012. PMID: 22178739 Free PMC article. - Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance.
Ma Y, Huang Y, Yan L, Gao M, Liu D. Ma Y, et al. Pharm Res. 2013 May;30(5):1447-57. doi: 10.1007/s11095-013-0986-7. Epub 2013 Feb 1. Pharm Res. 2013. PMID: 23371517 Free PMC article.
References
- Miettinen, H., Lehto, S., Salomaa, V., Mahonen, M., Niemela, M., Haffner, S. M., Pyorala, K. & Tuomilehto, J. (1998) Diabetes Care 21, 69–75. - PubMed
- Norhammar, A., Tenerz, A., Nilsson, G., Hamsten, A., Efendic, S., Ryden, L. & Malmberg, K. (2002) Lancet 359, 2140–2144. - PubMed
- The Diabetes Control and Complications Trial Research Group (1993) N. Engl. J. Med. 329, 977–986. - PubMed
- UK Prospective Diabetes Study Group (1998) Lancet 352, 837–853. - PubMed
- Ruderman, N. B. (1975) Annu. Rev. Med. 26, 245–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL068445/HL/NHLBI NIH HHS/United States
- HL68445/HL/NHLBI NIH HHS/United States
- GW07185/GW/GAS NIH HHS/United States
- HL30568/HL/NHLBI NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous