Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition - PubMed (original) (raw)
Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition
Damian Medici et al. Mol Biol Cell. 2006 Apr.
Abstract
Transforming growth factor beta 1 (TGF-beta1) has been shown to induce epithelial-mesenchymal transition (EMT) during various stages of embryogenesis and progressive disease. This alteration in cellular morphology is typically characterized by changes in cell polarity and loss of adhesion proteins such as E-cadherin. Here we demonstrate that EMT is associated with loss of claudin-1, claudin-2, occludin, and E-cadherin expression within 72 h of exposure to TGF-beta1 in MDCKII cells. It has been suggested that this expression loss occurs through TGF-beta1 in a Smad-independent mechanism, involving MEK and PI3K pathways, which have previously been shown to induce expression of the Snail (SNAI-1) gene. Here we show that these pathways are responsible for loss of tight junctions and a partial loss of E-cadherin. However, our results also demonstrate that a complete loss of E-cadherin and transformation to the mesenchymal phenotype are dependent on Smad signaling, which subsequently stimulates formation of beta-catenin/LEF-1 complexes that induce EMT.
Figures
Figure 1.
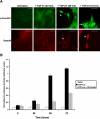
TGF-β1 stimulates Smad, MEK, and PI3K pathways in MDCKII cells. (A) Immuocytochemistry demonstrated TGF-β1 (10 ng/ml) induced Smad signaling via nuclear translocation (arrows) of p-Smad2/3 and Smad4 within 48 h of treatment (scale bar, 20 μm). (B) Smad transcriptional activity was confirmed by a p3TP-Lux reporter gene construct, with significant luciferase activity correlating with the nuclear localized Smad proteins. Addition of a DN Smad4 construct significantly inhibited p3TP-Lux activity (p < 0.04).
Figure 2.
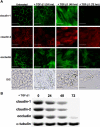
TGF-β1 suppresses tight junction proteins and promotes a clear change in cell morphology. (A) Expression and localization of the tight junction proteins claudin-1, claudin-2, and occludin were assessed via immunostaining, demonstrating losses of these molecules in the cell membrane within 24 h of treatment with TGF-β1. The epithelial nature of these cells changed dramatically into fibroblastlike morphology within 48 h (scale bar, 20 μm). (B) Decreases in expression levels of claudin-1, claudin-2, and occludin were confirmed via Western blotting, with nearly complete loss at 72 h.
Figure 3.
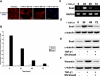
TGF-β1 promotes an epithelial-mesenchymal transition. (A) Expression levels of E-cadherin were observed by immunocytochemistry in MDCKII cells exposed to TGF-β1. Most of the E-cadherin protein was repressed at 48 h, with no detection at 72 h (scale bar, 20 μm). (B) These results were confirmed using a pGL3-E-cad-Lux reporter gene construct, with ∼40-50% of E-cadherin promoter activity was reduced within 24 h of treatment, followed by nearly full repression at 48-72 h (p < 0.017). (C) RT-PCR analysis showed levels of the E-cadherin repressor Snail peak at 24 h posttreatment, with a steady decline in expression through 72 h. (D) Immunoblotting demonstrated that Snail protein expression followed the same pattern as mRNA expression. (E) Additional immunoblotting for Snail at 24 h posttreatment showed that DN Smad4 had no effect on expression levels, suggesting that this mechanism is Smad-independent. (F) To confirm EMT, expression of the mesenchymal marker Vimentin was observed via immunoblotting in cells treated with TGF-β1 for 72 h. This expression increase was found to be Smad-dependent, as DN Smad4 prevented increased levels of Vimentin (scale bar, 20 μm).
Figure 4.
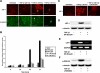
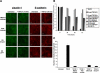
β-Catenin/LEF-1 signaling correlates with the transition to mesenchyme. (A) Nuclear localization (arrows) of β-catenin and LEF-1 were observed via immunostaining within 48 h of treatment with TGF-β1 (scale bar, 20 μm). (B) LEF-1 transcriptional activity was shown to correspond to the previously observed nuclear localization (A) using a pTOPFLASH-Lux reporter gene construct, with pFOPFLASH-Lux used as a negative control. DN Smad4 and DN LEF-1 significantly inhibited pTOPFLASH luciferase activity (p < 0.024). (C) The TGF-β1-induced LEF-1 expression observed via immunostaining was Smad-dependent, as we showed that expression was inhibited in cells containing a DN Smad4 construct (scale bar, 20 μm). These results were further confirmed by conducting (D) immunoblotting and (E) RT-PCR under the same conditions. (F) TGF-β1 was also shown to assist formation of the β-catenin/LEF-1 transcription complexes by preventing degradation of cytoplasmic β-catenin. We observed via immunoblotting that TGF-β1 promotes phosphorylation of GSK-3β within 24 h. This up-regulation of p-GSK-3β in inhibited in cells treated with a PI3K inhibitor, suggesting that this pathway is responsible for the associated phosphorylation.
Figure 5.
MEK and PI3K pathways suppress tight junctions, but full repression of adherens junctions and stimulation of EMT are dependent on Smad and β-catenin/LEF-1 signaling. (A) Expression of claudin-1 and E-cadherin was observed via immunocytochemistry in the presence of various inhibitors to assess the signaling pathways that control loss of tight junctions and adherens junctions during TGF-β-induced EMT. MEK1/2 and PI3K inhibitors prevented loss of claudin-1, whereas DN Smad4 and DN LEF-1 constructs did not. However, all inhibitors prevented loss of E-cadherin, suggesting that cooperation between all of these pathways is responsible for loss of adherens junctions and the transformation to mesenchyme (scale bar, 20 μm). (B) E-cadherin promoter activity was measured using the pGL3-E-cad reporter gene construct under the same conditions and showed that MEK and PI3K pathways correlate with expression of Snail. The later pathways of Smad and β-catenin/LEF-1 confer full repression of E-cadherin (p < 0.012). (C) Cell invasion into collagen gels was assessed showing high levels of invasion upon treatment with TGF-β1. The addition of MEK1/2 inhibitor, PI3K inhibitor, DNSmad4, or DN LEF-1 greatly decreased levels of cell invasion.
Figure 6.
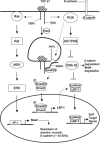
A schematic representation of the proposed TGF-β1 signaling mechanism that promotes EMT. Early signaling through Ras-Raf-MEK-ERK-AP-1 causes up-regulation in Snail expression. Snail, a well-known repressor of junction proteins, then inhibits expression of claudin-1, claudin-2, and occludin. Snail also provides partial loss of E-cadherin, thus decreasing the level of substrate for β-catenin. Further stabilization of cytoplasmic β-catenin is achieved through PI3K signaling. Molecules downstream of PI3K such as AKT phosphorylate and deactivate p-GSK-3β, which is responsible for degrading both β-catenin and Snail through the ubiquitin proteosome pathway. Smad signaling, controlled through endocytosis of TGF-β receptor complex, promotes transcription of LEF-1, an alternative substrate for β-catenin. On forming β-catenin/LEF-1 complexes, these molecules will then act to transcribe genes that will induce EMT.
Similar articles
- Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3.
Medici D, Hay ED, Olsen BR. Medici D, et al. Mol Biol Cell. 2008 Nov;19(11):4875-87. doi: 10.1091/mbc.e08-05-0506. Epub 2008 Sep 17. Mol Biol Cell. 2008. PMID: 18799618 Free PMC article. - The transcription factor LEF-1 induces an epithelial-mesenchymal transition in MDCK cells independent of β-catenin.
Kobayashi W, Ozawa M. Kobayashi W, et al. Biochem Biophys Res Commun. 2013 Dec 6;442(1-2):133-8. doi: 10.1016/j.bbrc.2013.11.031. Epub 2013 Nov 19. Biochem Biophys Res Commun. 2013. PMID: 24269234 - Transforming growth factor-beta signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis.
Nawshad A, Lagamba D, Polad A, Hay ED. Nawshad A, et al. Cells Tissues Organs. 2005;179(1-2):11-23. doi: 10.1159/000084505. Cells Tissues Organs. 2005. PMID: 15942189 Review. - Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions.
Doble BW, Woodgett JR. Doble BW, et al. Cells Tissues Organs. 2007;185(1-3):73-84. doi: 10.1159/000101306. Cells Tissues Organs. 2007. PMID: 17587811 Review.
Cited by
- New potential therapeutic targets to combat epithelial tumor invasion.
Peinado H, Cano A. Peinado H, et al. Clin Transl Oncol. 2006 Dec;8(12):851-7. doi: 10.1007/s12094-006-0148-z. Clin Transl Oncol. 2006. PMID: 17169758 Review. - Early bacterial colonization induces toll-like receptor-dependent transforming growth factor beta signaling in the epithelium.
Beisswenger C, Lysenko ES, Weiser JN. Beisswenger C, et al. Infect Immun. 2009 May;77(5):2212-20. doi: 10.1128/IAI.01224-08. Epub 2009 Mar 2. Infect Immun. 2009. PMID: 19255194 Free PMC article. - Anchoring junctions as drug targets: role in contraceptive development.
Mruk DD, Silvestrini B, Cheng CY. Mruk DD, et al. Pharmacol Rev. 2008 Jun;60(2):146-80. doi: 10.1124/pr.107.07105. Epub 2008 May 15. Pharmacol Rev. 2008. PMID: 18483144 Free PMC article. Review. - HPV16-E6 Oncoprotein Activates TGF-β and Wnt/_β_-Catenin Pathways in the Epithelium-Mesenchymal Transition of Cataracts in a Transgenic Mouse Model.
Rodríguez-Uribe G, Serafín-Higuera N, Damian-Morales G, Cortés-Malagón EM, García-Hernández V, Verdejo-Torres O, Campos-Blázquez JP, Trejo-Muñoz CR, Contreras RG, Ocadiz-Delgado R, Palacios-Reyes C, Lambert PF, Griep AE, Mancilla-Percino T, Escobar-Herrera J, Álvarez-Ríos E, Ugarte-Briones C, Moreno J, Gariglio P, Bonilla-Delgado J. Rodríguez-Uribe G, et al. Biomed Res Int. 2018 May 16;2018:2847873. doi: 10.1155/2018/2847873. eCollection 2018. Biomed Res Int. 2018. PMID: 29888254 Free PMC article. - RGC-32 mediates transforming growth factor-beta-induced epithelial-mesenchymal transition in human renal proximal tubular cells.
Huang WY, Li ZG, Rus H, Wang X, Jose PA, Chen SY. Huang WY, et al. J Biol Chem. 2009 Apr 3;284(14):9426-32. doi: 10.1074/jbc.M900039200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19158077 Free PMC article.
References
- Akhurst, R. J., and Derynck, R. (2001). TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 11, S44-S51. - PubMed
- Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., Portillo, F., and Nieto, M. A. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76-83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials