Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH - PubMed (original) (raw)
Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH
Giulia Taraboletti et al. Neoplasia. 2006 Feb.
Abstract
Tumor angiogenesis is regulated by a dynamic cross-talk between tumor cells and the host microenvironment. Because membrane vesicles shed by tumor cells are known to mediate several tumor-host interactions, we determined whether vesicles might also stimulate angiogenesis. Vesicles shed by human ovarian carcinoma cell lines CABA I and A2780 stimulated the motility and invasiveness of endothelial cells in vitro. Enzyme-linked immunosorbent assay and Western blot analysis revealed relevant amounts of vascular endothelial growth factor (VEGF) and the two matrix metalloproteinases MMP-2 and MMP-9, but not fibroblast growth factor-2, contained in shed vesicles. An A2780 cell-derived clone transfected to overexpress VEGF shed the same amount of vesicles as did a control clone, but contained significantly more VEGF within the vesicles. Despite a greater amount of VEGF in vesicles of the overexpressing clone, vesicles of both clones stimulated endothelial cell motility to comparable levels, suggesting that VEGF was stored within the vesicle and was unavailable. Only following vesicle burst induced by acidic pH (a characteristic of the tumor microenvironment) was VEGF released, leading to significantly higher stimulation of cell motility. Thus, tumor-shed membrane vesicles carry VEGF and release it in a bioactive form in conditions typical of the tumor microenvironment.
Figures
Figure 1
Scanning electron micrograph of CABA I (A) and A2780 (B) cell vesicle shedding.
Figure 2
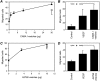
Effect of tumor cell-shed vesicles on endothelial cell motility and invasiveness. HUVEC motility (A and C) was tested in the Boyden chamber using isolated vesicles shed by human ovarian carcinoma cells CABA I (A; triangles) and A2780 (C; triangles) used as attractants. VEGF (10 ng/ml; squares) was used as a reference stimulus. In the invasion assay (B and D), HUVECs were stimulated by vesicles (5 µg) shed by CABA I (B) or A2780 (D), or by VEGF (10 ng/ml) used as a reference stimulus. Data (mean and SD of triplicates) represent the number of cells that migrated in 10 high-power fields (representative of two to four experiments). *P ≤ .05.
Figure 3
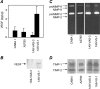
Molecular characterization of vesicles shed by human ovarian carcinoma cell lines and transfected variants. (A) ELISA analysis of VEGF in vesicles. (B) Western blot analysis of vesicle-associated VEGF. (C) Zymographic analysis of vesicle-associated gelatinases. (D) Reverse zymographic analysis of vesicle-associated TIMP-1 and TIMP-2. Experiments were conducted as described in Materials and Methods.
Figure 4
Stimulation of endothelial cell motility by shed vesicles. (A) Vesicles shed from 1A9-VAS-3 (circles) and 1A9-VS-1 (triangles) were isolated and tested for their ability to stimulate HUVEC motility (see Figure 2). (B) Vesicles isolated from 1A9-VS-1 cells were resuspended in either PBS (open triangles) or water (filled triangles), and, after correction of molarity, tested for motogenic activity. Data (mean and SD of triplicates) represent the number of cells that migrated in 10 high-power fields.
Figure 5
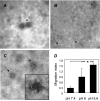
Effect of pH on vesicle integrity and activity. Freshly isolated 1A9-VS-1-derived vesicle pellets were resuspended in buffers at the indicated pH and analyzed by TEM with negative staining for changes in morphology (A–C) and motogenic activity (D). (A) Pelleted vesicles resuspended in PBS (pH 7.4), showing intact rounded vesicle structures. (B) Pelleted vesicles resuspended in buffer at pH 6.0, showing both intact vesicles (short arrow) and small membrane portions derived from broken vesicles (arrows). (C) Pelleted vesicles resuspended in buffer at pH 5.6, revealing no intact vesicles and only membrane fragments (arrows), often aggregated in amorphous masses (inset). (D) Chemotactic activity for HUVECs by vesicles derived from 1A9-VS-1 cells and resuspended in buffers at the indicated pH (neutralized before the assay). Data (mean and SD of triplicates) are expressed as migration index (see Materials and Methods) (representative of three experiments). *P ≤ .05. Scale bars, 1 µm (A–C).
Similar articles
- Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells.
Millimaggi D, Mari M, D'Ascenzo S, Carosa E, Jannini EA, Zucker S, Carta G, Pavan A, Dolo V. Millimaggi D, et al. Neoplasia. 2007 Apr;9(4):349-57. doi: 10.1593/neo.07133. Neoplasia. 2007. PMID: 17460779 Free PMC article. - Shedding of membrane vesicles by tumor and endothelial cells.
Dolo V, D'Ascenzo S, Giusti I, Millimaggi D, Taraboletti G, Pavan A. Dolo V, et al. Ital J Anat Embryol. 2005;110(2 Suppl 1):127-33. Ital J Anat Embryol. 2005. PMID: 16101030 - Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer.
Yabushita H, Shimazu M, Noguchi M, Kishida T, Narumiya H, Sawaguchi K, Noguchi M. Yabushita H, et al. Oncol Rep. 2003 Jan-Feb;10(1):89-95. Oncol Rep. 2003. PMID: 12469150 - Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer.
Zhang L, Yang N, Park JW, Katsaros D, Fracchioli S, Cao G, O'Brien-Jenkins A, Randall TC, Rubin SC, Coukos G. Zhang L, et al. Cancer Res. 2003 Jun 15;63(12):3403-12. Cancer Res. 2003. PMID: 12810677 - CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers.
Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. Kryczek I, et al. Cancer Res. 2005 Jan 15;65(2):465-72. Cancer Res. 2005. PMID: 15695388
Cited by
- The role of extracellular vesicles in cholangiocarcinoma tumor microenvironment.
Zhang N, Shu L, Liu Z, Shi A, Zhao L, Huang S, Sheng G, Yan Z, Song Y, Huang F, Tang Y, Zhang Z. Zhang N, et al. Front Pharmacol. 2024 Jan 10;14:1336685. doi: 10.3389/fphar.2023.1336685. eCollection 2023. Front Pharmacol. 2024. PMID: 38269274 Free PMC article. Review. - Gelatinases, endonuclease and Vascular Endothelial Growth Factor during development and regression of swine luteal tissue.
Ribeiro LA, Turba ME, Zannoni A, Bacci ML, Forni M. Ribeiro LA, et al. BMC Dev Biol. 2006 Nov 30;6:58. doi: 10.1186/1471-213X-6-58. BMC Dev Biol. 2006. PMID: 17137503 Free PMC article. - ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles.
Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. Muralidharan-Chari V, et al. Curr Biol. 2009 Dec 1;19(22):1875-85. doi: 10.1016/j.cub.2009.09.059. Epub 2009 Nov 5. Curr Biol. 2009. PMID: 19896381 Free PMC article. - An Overview of Epithelial-to-Mesenchymal Transition and Mesenchymal-to-Epithelial Transition in Canine Tumors: How Far Have We Come?
Armando F, Mazzola F, Ferrari L, Corradi A. Armando F, et al. Vet Sci. 2022 Dec 28;10(1):19. doi: 10.3390/vetsci10010019. Vet Sci. 2022. PMID: 36669020 Free PMC article. Review. - Paracrine induction of endothelium by tumor exosomes.
Hood JL, Pan H, Lanza GM, Wickline SA; Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN). Hood JL, et al. Lab Invest. 2009 Nov;89(11):1317-28. doi: 10.1038/labinvest.2009.94. Epub 2009 Sep 28. Lab Invest. 2009. PMID: 19786948 Free PMC article.
References
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous