Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1 - PubMed (original) (raw)
Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1
Ori Nevo et al. Am J Physiol Regul Integr Comp Physiol. 2006 Oct.
Abstract
Elevated expression of soluble vascular endothelial growth factor receptor-1 (sFlt-1) in preeclampsia plays a major role in the pathogenesis of this serious disorder of human pregnancy. Although reduced placental oxygenation is thought to be involved in the pathogenesis of preeclampsia, it is unclear how oxygen regulates placental sFlt-1 expression. The aims herein were to investigate sFlt-1 expression in in vivo and in vitro physiological and pathological models of human placental hypoxia and to understand the role of hypoxia inducible factor-1 (HIF-1) in regulating the expression of this molecule. sFlt-1 expression in placental villi was significantly increased under physiological low oxygen conditions in early first-trimester and in high-altitude placentae, as well as in pathological low oxygen conditions, such as preeclampsia. In high-altitude and in preeclamptic tissue, sFlt-1 localized within villi to perivascular regions, the syncytiotrophoblast layer, and syncytial knots. In first-trimester villous explants, low oxygen, but not hypoxia-reoxygenation (HR), increased sFlt-1 expression. Moreover, exposure of villous explants to dimethyloxalyl-glycin, a pharmacological inhibitor of prolyl-hydroxylases, which mimics hypoxia by increasing HIF-1alpha stability, increased sFlt-1 expression. Conversely, HIF-1alpha knockdown using antisense oligonucleotides, decreased sFlt-1 expression. In conclusion, placental sFlt-1 expression is increased by both physiologically and pathologically low levels of oxygen. This oxygen-induced effect is mediated via the transcription factor HIF-1. Low oxygen levels, as opposed to intermittent oxygen tension (HR) changes, play an important role in regulating sFlt-1 expression in the developing human placenta and hence may contribute to the development of preeclampsia.
Figures
Fig. 1.
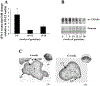
sFlt-1 expression during placental development. A: expression of sFlt-1 transcript in early first-trimester placental samples vs. later gestation as assessed by real-time PCR analysis (n = 11 for each gestational age tested); *P < 0.05, 5–9 wk vs. 10–12 wk. B: representative sFlt-1 immunoblot of first and early second-trimester samples. 5–9 wk, n = 5; 10–12 wk, n = 5; 13–18 wk, n = 7. C: immunolocalization of sFlt-1 in first-trimester tissue. Dark gray staining represents positive immunoreactivity. (CT, cytotrophoblasts; ST, syncytiotropho-blast, SK, syncytial knot; S, stroma). All values are represented as the means ± SE.
Fig. 2.
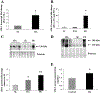
sFlt-1 expression in high-altitude and preeclamptic placental samples. A: expression of sFlt-1 mRNA in high-altitude (HA) vs. sea level (SL) samples assessed by real-time PCR analysis. HA, n = 15, SL, n = 12. *P < 0.001, HA vs. SL. B: fold change in the transcript level of sFlt-1 in early severe preeclampsia (PE, n = 18) compared with age-matched controls (AMC, n = 12) assessed by real-time PCR. *P < 0.01, PE vs. term control (TC) and vs. preterm control (PTC), respectively. C, top: representative sFlt-1 Western blot analysis in placental tissues from high-altitude (HA, n = 14) and preeclamptic pregnancies (PE, n = 5), relative to SL controls (n = 11). Bottom: sFlt-1 protein densitometric analysis in HA, PE, and control SL. Data are normalized vs. sea-level samples. *P < 0.01, HA vs. SL; **P < 0.01, PE vs. HA and SL. D: representative Flt-1 Western blot analsis in placental tissues from HA (n = 6) and preeclamptic pregnancies (PE, n = 3), relative to SL controls (n = 5). E: relative levels of circulating sFlt-1 protein in serum of pregnant patients from HA and lower altitude (control) near term (n = 16). Values are mean ± SE. *P < 0.05, HA vs. control.
Fig. 3.
Immunolocalization of sFlt-1 in representative HA, PE, and SL placental tissue. a_–_c: sea level controls, n = 10. d_–_f: high-altitude samples, n = 8. g_–_k: early preeclampsia samples, n = 6. l: negative control (no 1° antibody). Dark gray staining represents positive sFlt-1 immunostaining. ET, endothelium; PV, perivascular.
Fig. 4.
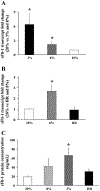
Effect of low oxygen and hypoxia-reoxygenation (HR) on sFlt-1 expression in first-trimester villous explants. A: expression of sFlt-1 mRNA in explants cultured at 3% and 8% vs. 20% O2 measured by qRT-PCR analysis, n = 7. *P < 0.05, 3% vs. 20% O2. B: real-time RT-PCR analysis of sFlt-1 mRNA in explants cultured in 20% and 8% O2 compared with HR conditions, n = 5. *P < 0.05, 8% vs. HR; *P < 0.05, 8% vs. 20% O2. C: sFlt-1 protein concentration measured by ELISA in conditioned media from first-trimester placental explants that were cultured in 20%, 8%, and 3% O2 compared with HR, n = 8. *P < 0.05, 3% vs. HR and 20%. Values are expressed as means ± SE of at least five separate experiments carried out in triplicate.
Fig. 5.
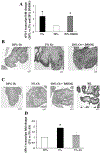
Effect of dimethyloxalyl-glycin (DMOG) and antisense oligonucleotides to hypoxia inducible factor (HIF-1α) on sFlt-1 expression in first-trimester placental explants. A: effect of DMOG treatment on sFlt-1 transcript in villous explants assessed by qRT-PCR, n = 3. *P < 0.05, 3% and 20% DMOG vs. 20% O2. B: effect of DMOG treatment on spatial localization of sFlt-1 protein in villous explants, n = 3. Dark gray staining represents positive immunoreactivity. C: TUNEL staining of villous explants and placental sample of early PE. Positive staining appears as black nuclear staining. D: effect of antisense oligonucleotides to HIF-1α (AS) on sFlt-1 mRNA expression in explants, n = 6. *P < 0.05, 3% O2 vs. 3% O2 +AS. Values are expressed as means ± SE of at least three separate experiments carried out in triplicate.
Similar articles
- Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women.
Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Rajakumar A, et al. Placenta. 2005 Aug;26(7):563-73. doi: 10.1016/j.placenta.2004.09.001. Placenta. 2005. PMID: 15993706 - Attenuation of VEGFR-2 expression by sFlt-1 and low oxygen in human placenta.
Nevo O, Lee DK, Caniggia I. Nevo O, et al. PLoS One. 2013 Nov 19;8(11):e81176. doi: 10.1371/journal.pone.0081176. eCollection 2013. PLoS One. 2013. PMID: 24260556 Free PMC article. - [Expression of hypoxia-inducible factor-1alpha, vascular endothelial growth factor and sFlt-1 in preeclampsia placenta].
Sun SG, Shen N, Zheng YH, Shang T. Sun SG, et al. Zhonghua Fu Chan Ke Za Zhi. 2006 Jul;41(7):440-4. Zhonghua Fu Chan Ke Za Zhi. 2006. PMID: 17083805 Chinese. - Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review.
Ahmed A, Dunk C, Ahmad S, Khaliq A. Ahmed A, et al. Placenta. 2000 Mar-Apr;21 Suppl A:S16-24. doi: 10.1053/plac.1999.0524. Placenta. 2000. PMID: 10831117 Review.
Cited by
- Linking placental ischemia and hypertension in preeclampsia: role of endothelin 1.
George EM, Granger JP. George EM, et al. Hypertension. 2012 Aug;60(2):507-11. doi: 10.1161/HYPERTENSIONAHA.112.194845. Epub 2012 May 7. Hypertension. 2012. PMID: 22566502 Free PMC article. No abstract available. - Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy.
Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Pringle KG, et al. Hum Reprod Update. 2010 Jul-Aug;16(4):415-31. doi: 10.1093/humupd/dmp046. Epub 2009 Nov 19. Hum Reprod Update. 2010. PMID: 19926662 Free PMC article. Review. - Evidence of sexual dimorphism in the placental function with severe preeclampsia.
Muralimanoharan S, Maloyan A, Myatt L. Muralimanoharan S, et al. Placenta. 2013 Dec;34(12):1183-9. doi: 10.1016/j.placenta.2013.09.015. Epub 2013 Sep 29. Placenta. 2013. PMID: 24140080 Free PMC article. - Enhancement of the HIF-1α/15-LO/15-HETE axis promotes hypoxia-induced endothelial proliferation in preeclamptic pregnancy.
Yuan D, Ran Y, Liu Q, Zhang Y, Li H, Li P, Zhu D. Yuan D, et al. PLoS One. 2014 May 5;9(5):e96510. doi: 10.1371/journal.pone.0096510. eCollection 2014. PLoS One. 2014. PMID: 24796548 Free PMC article. - Modelling preeclampsia: a comparative analysis of the common human trophoblast cell lines.
Zhao J, Chow RP, McLeese RH, Hookham MB, Lyons TJ, Yu JY. Zhao J, et al. FASEB Bioadv. 2020 Nov 21;3(1):23-35. doi: 10.1096/fba.2020-00057. eCollection 2021 Jan. FASEB Bioadv. 2020. PMID: 33521587 Free PMC article.
References
- American College of Obstetrics and Gynecology. Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Int J Gynaecol Obstet 77: 67–75, 2002. - PubMed
- Ahmad S and Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004. - PubMed
- Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, Kaufmann S, Walters CE, Jackson A, Eves P, Linton G, Keen J, Walker JJ, and Selby PJ. Evidence for the existence of a novel preg nancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod 4: 377–386, 1998. - PubMed
- Benirschke K Recent trends in chorangiomas, especially those of multiple and recurrent chorangiomas. Pediatr Dev Pathol 2: 264–269, 1999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources