Viruses take center stage in cellular evolution - PubMed (original) (raw)
Viruses take center stage in cellular evolution
Jean-Michel Claverie. Genome Biol. 2006.
Abstract
The origins of viruses are shrouded in mystery, but advances in genomics and the discovery of highly complex giant DNA viruses have stimulated new hypotheses that DNA viruses were involved in the emergence of the eukaryotic cell nucleus, and that they are worthy of being considered as living organisms.
Figures
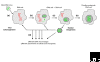
Figure 1
A possible iterative scenario for viral eukaryogenesis and nuclear viriogenesis. (a) A primitive DNA virus (a bacteriophage ancestor) gets trapped within an RNA cell and becomes a primitive nucleus. (b) Cellular genes are progressively recruited to the enlarging nucleus because of the selective advantages of DNA biochemistry. (c) For a while this situation remains unstable and reversible, allowing new 'pre-eukaryotic viruses' to be created. These viruses reinfect other cells at various stages of this iterative process. (d) This hypothetical scheme provides a mechanism for the emergence of various overlapping but not monophyletic virus lineages as well as for the rapid reassortment of genes from the viral and cellular pools before they reach their 'Darwinian threshold' [29], that is, (e) the evolution of a stable eukaryotic cell with a fully DNA nuclear genome.
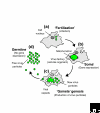
Figure 2
What is a virus? The life cycle of a complex dsDNA virus (for example NCLDVs) is shown. (a) A virus particle infects the cell and releases its DNA into the cytoplasm. (b) The viral DNA replicates and capsid proteins are synthesized within a 'virus factory' in the cytoplasm to which are recruited cellular ribosomes and the protein-synthesis machinery, as well as mitochondria to provide ATP. (c) New infectious viral particles are produced (while the nucleus is fading) and (d) released from the cell to begin another round of infection and replication. I propose that the true nature of complex eukaryotic dsDNA viruses is found in the transient virus factory they produce at each generation, rather than in the reproductive virus particle with which they have been equated. The virus factory is proposed to represent the result of the progressive reductive evolution of an obligate parasitic cellular organism, committed to the viral evolutionary pathway by the loss of a functional translation machinery. For a viral organism, the virus factory exhibits all the properties of the soma, in which genes are expressed, while the particle state corresponds to the germline (sensu August Weismann [28]) which remains unchanged. If we follow this line of thought, one might think of infection as being analogous with fertilization and the production of new virus particles as being akin to the formation of gametes.
Similar articles
- Evolutionary genomics of nucleo-cytoplasmic large DNA viruses.
Iyer LM, Balaji S, Koonin EV, Aravind L. Iyer LM, et al. Virus Res. 2006 Apr;117(1):156-84. doi: 10.1016/j.virusres.2006.01.009. Epub 2006 Feb 21. Virus Res. 2006. PMID: 16494962 Review. - Convergent mechanisms of genome evolution of large and giant DNA viruses.
Filée J, Chandler M. Filée J, et al. Res Microbiol. 2008 Jun;159(5):325-31. doi: 10.1016/j.resmic.2008.04.012. Epub 2008 May 10. Res Microbiol. 2008. PMID: 18572389 - Iridovirus homologues of cellular genes--implications for the molecular evolution of large DNA viruses.
Tidona CA, Darai G. Tidona CA, et al. Virus Genes. 2000;21(1-2):77-81. Virus Genes. 2000. PMID: 11022791 Review. - Diversification of giant and large eukaryotic dsDNA viruses predated the origin of modern eukaryotes.
Guglielmini J, Woo AC, Krupovic M, Forterre P, Gaia M. Guglielmini J, et al. Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19585-19592. doi: 10.1073/pnas.1912006116. Epub 2019 Sep 10. Proc Natl Acad Sci U S A. 2019. PMID: 31506349 Free PMC article. - Endogenous DNA viruses take center stage in eukaryotic genome evolution.
Moniruzzaman M, Aylward FO. Moniruzzaman M, et al. Proc Natl Acad Sci U S A. 2023 May 23;120(21):e2305212120. doi: 10.1073/pnas.2305212120. Epub 2023 May 15. Proc Natl Acad Sci U S A. 2023. PMID: 37186839 Free PMC article. No abstract available.
Cited by
- Giant virus with a remarkable complement of genes infects marine zooplankton.
Fischer MG, Allen MJ, Wilson WH, Suttle CA. Fischer MG, et al. Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19508-13. doi: 10.1073/pnas.1007615107. Epub 2010 Oct 25. Proc Natl Acad Sci U S A. 2010. PMID: 20974979 Free PMC article. - The ancient Virus World and evolution of cells.
Koonin EV, Senkevich TG, Dolja VV. Koonin EV, et al. Biol Direct. 2006 Sep 19;1:29. doi: 10.1186/1745-6150-1-29. Biol Direct. 2006. PMID: 16984643 Free PMC article. - RdRp (RNA-dependent RNA polymerase): A key target providing anti-virals for the management of various viral diseases.
Pathania S, Rawal RK, Singh PK. Pathania S, et al. J Mol Struct. 2022 Feb 15;1250:131756. doi: 10.1016/j.molstruc.2021.131756. Epub 2021 Oct 17. J Mol Struct. 2022. PMID: 34690363 Free PMC article. Review. - Marine mimivirus relatives are probably large algal viruses.
Monier A, Larsen JB, Sandaa RA, Bratbak G, Claverie JM, Ogata H. Monier A, et al. Virol J. 2008 Jan 23;5:12. doi: 10.1186/1743-422X-5-12. Virol J. 2008. PMID: 18215256 Free PMC article. - The polyadenylation site of Mimivirus transcripts obeys a stringent 'hairpin rule'.
Byrne D, Grzela R, Lartigue A, Audic S, Chenivesse S, Encinas S, Claverie JM, Abergel C. Byrne D, et al. Genome Res. 2009 Jul;19(7):1233-42. doi: 10.1101/gr.091561.109. Epub 2009 Apr 29. Genome Res. 2009. PMID: 19403753 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources