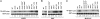Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance - PubMed (original) (raw)
Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance
Gürol Tuncman et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The c-Jun N-terminal kinases (JNKs) are key regulators of inflammation and interfere with insulin action in cultured cells and whole animals. Obesity increases total JNK activity, and JNK1, but not JNK2, deficiency results in reduced adiposity and improved insulin sensitivity. Interestingly, a higher-than-normal level of JNK activation is observed in Jnk2(-/-) mice, particularly in the liver, indicating an interaction between the isoforms that might have masked the metabolic activity of JNK2 in isolated mutant mice. To address the role of the JNK2 isoform in metabolic homeostasis, we intercrossed Jnk1(-/-) and Jnk2(-/-) mice and examined body weight and glucose metabolism in the resulting mutant allele combinations. Among all of the viable genotypes examined, we observed only reduced body weight and increased insulin sensitivity in Jnk1(-/-) and Jnk1(+/-)Jnk2(-/-) mice. These two groups of mice also exhibited reduced total JNK activity and cytokine expression in liver tissue compared with all other genotypes examined. These data indicate that the JNK2 isoform is also involved in metabolic regulation, but its function is not obvious when JNK1 is fully expressed because of regulatory crosstalk between the two isoforms.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
Regulation of JNK activity by obesity and its modulation by isolated JNK1 and JNK2 deficiencies. Total JNK activity was determined in liver, white adipose tissue (WAT), and muscle tissues of WT, ob/ob, _ob/ob_-_TNFR_−/−(_p55_−/−_p75_−/−), _Jnk1_−/−, and _Jnk2_−/− mice. ob/ob, genetic model for leptin deficiency. Numbers below the immunoblots represent fold increase in c-Jun N-terminal phosphorylation. 1, activity in lean WT.
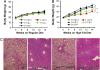
Fig. 2.
Body-weight regulation and fatty liver in different Jnk genotypes on a high-fat diet. (A and B) Starting at 3 weeks of age, all progeny from the intercrosses between Jnk1+/− and Jnk2+/− were placed on a regular (A) and high-fat (B) diet and were monitored for their body weight. Five genotypes are shown in these graphs. ∗∗, statistical significance P < 0.01. (C) Liver sections prepared from Jnk1+/+Jnk2+/+, Jnk1+/−_Jnk2_−/−, Jnk1+/+_Jnk2_−/−, and Jnk1_−/−_Jnk2+/+ mice on with a high-fat/high-caloric diet for 17 weeks. Sections were photographed at ×100 after staining with hematoxylin/eosin.
Fig. 3.
Biochemical analyses of serum in different Jnk genotypes. Serum samples were collected after an overnight fast from mice of the indicated genotypes after 14 weeks on a high-fat diet. Triglyceride (A), cholesterol (B), insulin (C), and glucose (D) levels were measured. ∗, statistical significance P < 0.05.
Fig. 4.
Comparison of insulin sensitivity among different Jnk genotypes. Systemic glucose metabolism and insulin sensitivity were studied by i.p. glucose and i.p. insulin tolerance tests performed on mice kept on a regular (A and C) and high-fat diet (B and D) at 16–17 weeks of age. AUC, area under the curve. ∗, statistical significance P < 0.01.
Fig. 5.
Total JNK activity and expression of inflammatory cytokines. Total JNK activity was determined in livers of Jnk1+/−_Jnk2_−/−, Jnk1_−/−_Jnk2+/+, and Jnk1+/+_Jnk2_−/− mice kept on a high-fat diet (A) along with their lean and obese WT controls at 17 weeks of age. Total RNA was extracted from the same liver samples, and IL-12 (B), MCP-1 (C), TNF-α (D), IL-6 (E), and MIF (F) mNA levels were quantified relative to that of 18S ribosomal RNA.
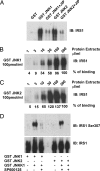
Fig. 6.
Binding of JNK1 and JNK2 to IRS-1 and its phosphorylation on Ser-307 residue. (A) GST pulldown of lysates (300 μg/ml) from HEK293T cells transiently expressing IRS-1 in the presence or absence of the competitive inhibitor of JNK substrate docking, Tat-JIP (5 μM). (B and C) GST-JNK1 (B) and GST-JNK2 (C) pulldown experiments using different amounts of extracts from IRS-1-expressing HEK293T cells. (D) GST-JNK1 and GST-JNK2 pulldown kinase assays were performed by using 300 μg/ml of protein lysates from IRS-1-expressing HEK293T cells in the presence or absence of the JNK inhibitor SP600125 (2 μM). IRS-1 Ser-307 phosphorylation was detected by immunoblotting with a phosphospecific antibody.
Similar articles
- Genetic deletion of JNK1 and JNK2 aggravates the DSS-induced colitis in mice.
Chromik AM, Müller AM, Körner J, Belyaev O, Holland-Letz T, Schmitz F, Herdegen T, Uhl W, Mittelkötter U. Chromik AM, et al. J Invest Surg. 2007 Jan-Feb;20(1):23-33. doi: 10.1080/08941930601126140. J Invest Surg. 2007. PMID: 17365404 - Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance.
Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Singh R, et al. Hepatology. 2009 Jan;49(1):87-96. doi: 10.1002/hep.22578. Hepatology. 2009. PMID: 19053047 Free PMC article. - Jnk1 Deficiency in Hematopoietic Cells Suppresses Macrophage Apoptosis and Increases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice.
Babaev VR, Yeung M, Erbay E, Ding L, Zhang Y, May JM, Fazio S, Hotamisligil GS, Linton MF. Babaev VR, et al. Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1122-31. doi: 10.1161/ATVBAHA.116.307580. Epub 2016 Apr 21. Arterioscler Thromb Vasc Biol. 2016. PMID: 27102962 Free PMC article. - Brain JNK and metabolic disease.
Nogueiras R, Sabio G. Nogueiras R, et al. Diabetologia. 2021 Feb;64(2):265-274. doi: 10.1007/s00125-020-05327-w. Epub 2020 Nov 16. Diabetologia. 2021. PMID: 33200240 Review. - JNK2: a negative regulator of cellular proliferation.
Sabapathy K, Wagner EF. Sabapathy K, et al. Cell Cycle. 2004 Dec;3(12):1520-3. doi: 10.4161/cc.3.12.1315. Epub 2004 Dec 18. Cell Cycle. 2004. PMID: 15611655 Review.
Cited by
- Medium-chain triglyceride ameliorates insulin resistance and inflammation in high fat diet-induced obese mice.
Geng S, Zhu W, Xie C, Li X, Wu J, Liang Z, Xie W, Zhu J, Huang C, Zhu M, Wu R, Zhong C. Geng S, et al. Eur J Nutr. 2016 Apr;55(3):931-40. doi: 10.1007/s00394-015-0907-0. Epub 2015 Apr 25. Eur J Nutr. 2016. PMID: 25911003 - C-Jun N-terminal kinase inhibitors: Structural insight into kinase-inhibitor complexes.
Duong MTH, Lee JH, Ahn HC. Duong MTH, et al. Comput Struct Biotechnol J. 2020 Jun 12;18:1440-1457. doi: 10.1016/j.csbj.2020.06.013. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32637042 Free PMC article. Review. - The immune system's involvement in obesity-driven type 2 diabetes.
Shu CJ, Benoist C, Mathis D. Shu CJ, et al. Semin Immunol. 2012 Dec;24(6):436-42. doi: 10.1016/j.smim.2012.12.001. Epub 2013 Jan 18. Semin Immunol. 2012. PMID: 23333525 Free PMC article. Review. - Mechanisms for insulin resistance: common threads and missing links.
Samuel VT, Shulman GI. Samuel VT, et al. Cell. 2012 Mar 2;148(5):852-71. doi: 10.1016/j.cell.2012.02.017. Cell. 2012. PMID: 22385956 Free PMC article. Review. - Insulin Resistance and Diabetes Mellitus in Alzheimer's Disease.
Burillo J, Marqués P, Jiménez B, González-Blanco C, Benito M, Guillén C. Burillo J, et al. Cells. 2021 May 18;10(5):1236. doi: 10.3390/cells10051236. Cells. 2021. PMID: 34069890 Free PMC article. Review.
References
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Science. 1993;259:87–91. - PubMed
- Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. Nature. 1997;389:610–614. - PubMed
- Ventre J., Doebber T., Wu M., Macnaul K., Stevens K., Pasparakis M., Kollias G., Moller D. E. Diabetes. 1997;46:1526–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R37 ES004151/ES/NIEHS NIH HHS/United States
- P30 DK040561-11/DK/NIDDK NIH HHS/United States
- ES04151/ES/NIEHS NIH HHS/United States
- R01 DK052539/DK/NIDDK NIH HHS/United States
- DK52539/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous