Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene - PubMed (original) (raw)
doi: 10.1086/507566. Epub 2006 Jul 31.
Jurg Ott, Sandra Barral, Pierre Bovet, Patricia Deppen, Fulvia Gheorghita, Marie-Louise Matthey, Josef Parnas, Martin Preisig, Michael Saraga, Alessandra Solida, Sally Timm, August G Wang, Thomas Werge, Michel Cuénod, Kim Quang Do
Affiliations
- PMID: 16909399
- PMCID: PMC1559555
- DOI: 10.1086/507566
Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene
Mirjana Tosic et al. Am J Hum Genet. 2006 Sep.
Abstract
Oxidative stress could be involved in the pathophysiology of schizophrenia, a major psychiatric disorder. Glutathione (GSH), a redox regulator, is decreased in patients' cerebrospinal fluid and prefrontal cortex. The gene of the key GSH-synthesizing enzyme, glutamate cysteine ligase modifier (GCLM) subunit, is strongly associated with schizophrenia in two case-control studies and in one family study. GCLM gene expression is decreased in patients' fibroblasts. Thus, GSH metabolism dysfunction is proposed as one of the vulnerability factors for schizophrenia.
Figures
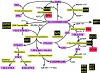
Figure 1.
GSH metabolic pathway. The genes for which expression was studied in fibroblast cultures from skin biopsies are highlighted. Black indicates no difference between controls and patients, and red indicates lower expression in patients compared with controls. These genes code for the enzymes highlighted in yellow. Substrates and products are highlighted in purple. Genes not directly involved in GSH metabolism but included in the expression study: Nrf1, Nrf2, and Nrf3 = NF-E2–related transcription factors 1, 2, and 3, respectively; xCT and 4F2 = genes encoding proteins involved in cystine/glutamate exchange; and MRP1 = multidrug resistance protein 1. ADP = adenosine diphospate; GSSG = glutathione disulfide; RSH = reduced thiols; RS = disulfide; N-AC-CYS-X = N-acetyl-cysteine conjugate; Pi = inorganic phosphate.
Figure 2.
Map of GCLM and GSS genomic structures, with the positions of SNP markers and results of two case-control studies. Tables show P values for each SNP in case-control studies of subjects from two independent populations, from Switzerland and Denmark. Values in italics indicate a significant difference, and the value in bold italics indicates a significant difference after correction for multiple testing.
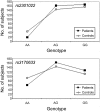
Figure 3.
Genotype distribution of the two most common SNPs at the GCLM gene in the controls and patients sampled from a Danish population. The graph shows a dominance of the G allele for both markers.
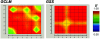
Figure 4.
GOLD plot of pairwise LD for 17 SNPs that belong to genes GCLM and GSS. The 17 SNPs used in association and linkage studies and graphically presented in figure 2 are labeled on both axes. The color differences represent regions of the local map that are in disequilibrium and the magnitude of that disequilibrium. Regions of high and low LD are presented in red and blue, respectively. GOLD plot shows very strong LD within each gene.
Similar articles
- No association between glutathione-synthesis-related genes and Japanese schizophrenia.
Hanzawa R, Ohnuma T, Nagai Y, Shibata N, Maeshima H, Baba H, Hatano T, Takebayashi Y, Hotta Y, Kitazawa M, Arai H. Hanzawa R, et al. Psychiatry Clin Neurosci. 2011 Feb;65(1):39-46. doi: 10.1111/j.1440-1819.2010.02157.x. Epub 2010 Nov 24. Psychiatry Clin Neurosci. 2011. PMID: 21105962 - Mutation screening of the glutamate cysteine ligase modifier (GCLM) gene in patients with schizophrenia.
Butticaz C, Werge T, Beckmann JS, Cuénod M, Do KQ, Rivolta C. Butticaz C, et al. Psychiatr Genet. 2009 Aug;19(4):201-8. doi: 10.1097/YPG.0b013e32832cef21. Psychiatr Genet. 2009. PMID: 19455074 - Genetic analysis of glutamate cysteine ligase modifier (GCLM) gene and schizophrenia in Han Chinese.
Ma J, Li DM, Zhang R, Yang XD, Gao CG, Lu SM, Wu SN, Wang L. Ma J, et al. Schizophr Res. 2010 Jun;119(1-3):273-4. doi: 10.1016/j.schres.2009.12.017. Epub 2010 Jan 12. Schizophr Res. 2010. PMID: 20061124 No abstract available. - Modulating GSH synthesis using glutamate cysteine ligase transgenic and gene-targeted mice.
Botta D, White CC, Vliet-Gregg P, Mohar I, Shi S, McGrath MB, McConnachie LA, Kavanagh TJ. Botta D, et al. Drug Metab Rev. 2008;40(3):465-77. doi: 10.1080/03602530802186587. Drug Metab Rev. 2008. PMID: 18642143 Review. - Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models.
Kulak A, Steullet P, Cabungcal JH, Werge T, Ingason A, Cuenod M, Do KQ. Kulak A, et al. Antioxid Redox Signal. 2013 Apr 20;18(12):1428-43. doi: 10.1089/ars.2012.4858. Epub 2012 Oct 12. Antioxid Redox Signal. 2013. PMID: 22938092 Review.
Cited by
- Schizophrenia and Glutathione: A Challenging Story.
Carletti B, Banaj N, Piras F, Bossù P. Carletti B, et al. J Pers Med. 2023 Oct 25;13(11):1526. doi: 10.3390/jpm13111526. J Pers Med. 2023. PMID: 38003841 Free PMC article. Review. - Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-blind, randomized, placebo-controlled trial.
Carmeli C, Knyazeva MG, Cuénod M, Do KQ. Carmeli C, et al. PLoS One. 2012;7(2):e29341. doi: 10.1371/journal.pone.0029341. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22383949 Free PMC article. Clinical Trial. - Striatal Glutathione in First-episode Psychosis Patients Measured In Vivo with Proton Magnetic Resonance Spectroscopy.
Reyes-Madrigal F, León-Ortiz P, Mao X, Mora-Durán R, Shungu DC, de la Fuente-Sandoval C. Reyes-Madrigal F, et al. Arch Med Res. 2019 May;50(4):207-213. doi: 10.1016/j.arcmed.2019.08.003. Epub 2019 Sep 26. Arch Med Res. 2019. PMID: 31499481 Free PMC article. - Mitochondria, Metabolism, and Redox Mechanisms in Psychiatric Disorders.
Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, Santos R. Kim Y, et al. Antioxid Redox Signal. 2019 Aug 1;31(4):275-317. doi: 10.1089/ars.2018.7606. Epub 2019 Feb 1. Antioxid Redox Signal. 2019. PMID: 30585734 Free PMC article. Review. - Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia.
Jiang Z, Cowell RM, Nakazawa K. Jiang Z, et al. Front Behav Neurosci. 2013 Sep 3;7:116. doi: 10.3389/fnbeh.2013.00116. Front Behav Neurosci. 2013. PMID: 24027504 Free PMC article. Review.
References
Web Resources
- International Classification of Diseases (ICD-10), http://www3.who.int/icd/currentversion/fr-icd.htm
- Online Mendelian Inheritance in Man (OMIM) http://www.ncbi.nlm.nih.gov/Omim/ (for schizophrenia)
- SNP Consortium, http://snp.cshl.org/
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials