The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport - PubMed (original) (raw)
. 2006 Oct 1;119(Pt 19):3944-57.
doi: 10.1242/jcs.03153. Epub 2006 Sep 5.
Affiliations
- PMID: 16954148
- PMCID: PMC1904490
- DOI: 10.1242/jcs.03153
The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport
Anna C Rutherford et al. J Cell Sci. 2006.
Abstract
The yeast gene fab1 and its mammalian orthologue Pip5k3 encode the phosphatidylinositol 3-phosphate [PtdIns(3)P] 5-kinases Fab1p and PIKfyve, respectively, enzymes that generates phosphatidylinositol 3,5-bisphosphate [PtdIns(3,5)P(2)]. A shared feature of fab1Delta yeast cells and mammalian cells overexpressing a kinase-dead PIKfyve mutant is the formation of a swollen vacuolar phenotype: a phenotype that is suggestive of a conserved function for these enzymes and their product, PtdIns(3,5)P(2), in the regulation of endomembrane homeostasis. In the current study, fixed and live cell imaging has established that, when overexpressed at low levels in HeLa cells, PIKfyve is predominantly associated with dynamic tubular and vesicular elements of the early endosomal compartment. Moreover, through the use of small interfering RNA, it has been shown that suppression of PIKfyve induces the formation of swollen endosomal structures that maintain their early and late endosomal identity. Although internalisation, recycling and degradative sorting of receptors for epidermal growth factor and transferrin was unperturbed in PIKfyve suppressed cells, a clear defect in endosome to trans-Golgi-network (TGN) retrograde traffic was observed. These data argue that PIKfyve is predominantly associated with the early endosome, from where it regulates retrograde membrane trafficking to the TGN. It follows that the swollen endosomal phenotype observed in PIKfyve-suppressed cells results primarily from a reduction in retrograde membrane fission rather than a defect in multivesicular body biogenesis.
Figures
Fig. 1
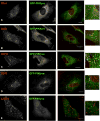
At low-level expression GFP-tagged PIKfyve is predominantly associated with the early endosome. (A–E) HeLa cells were transiently transfected with GFP-PIKfyve and, after 24 hours, cells were fixed and stained for the early endosomal markers EEA1 (A) and SNX1 (B), internalised EGF receptor 10 minutes after EGF addition (C), and the late endosomal markers CD63 (D) and LAMP1 (E). Images are single, 0.1 μm confocal sections and are representative of more than ten imaged cells in each case. Bars, 10 μm. Boxed area in A–E is shown enlarged on the right.
Fig. 2
The GFP-PIKfyve-positive early endosome is a highly dynamic tubular-vesicular compartment. (A,B) One HeLa cell expressing low levels of GFP-PIKfyve was imaged at one frame per second for a period of 10 minutes (A). Boxed areas indicate regions of interest detailed in C and D. Subsequently, data from a 100-second period were selected and each individual image was analysed (see Materials and Methods) to obtain a map of spatial temporal behaviour of the PIKfyve-labelled early endosome (B). Coloured asterisks are used to indicate the time at which the chosen vesicle first appeared in the image plane. Subsequent movements of >0.4 μm were followed over time. As indicated in the timescale bar, each colour corresponds to a specific 5-second period during the image window. The resulting 2D image gives an overview of both, the speed and directionality of the PIKfyve-positive punctae. The trafficking map revealed a directional trend, with several PIKfyve-positive vesicles tracking towards the perinuclear and/or TGN region. (C) The progress of one such vesicle is highlighted, with the first frame being denominated time point zero. (D,E) Live cell imaging also revealed that (D) PIKfyve-labelled vesicles (arrows) were able to undergo homotypic fusion (arrowhead), and that (E) the PIKfyve-labelled tubules were able to emminate from the vesicular elements of the PIKfyve-labelled compartment.
Fig. 3
Internalised Alexa568-Tf and TxR-EGF are transported through the GFP-PIKfyve-labelled early endosome. (A-D) HeLa cells were transiently transfected with pEGFPC1-PIKfyve for 22 hours and serum-starved for 3 hours prior to live cell imaging. Cells were incubated with either (A,B) 200 ng/ml TxR-EGF or (C,D) 20 μg/ml Alexa568-Tf. TxR-EGF reached the GFP-PIKfyve-positive compartment after 15 minutes, and is not transported along GFP-PIKfyve-positive tubules but remains in the endosomal body. Series of frames (B) depicting a GFP-PIKfyve-positive tubule (arrow) exiting an endosome positive for GFP-PIKfyve and TxR-EGF (arrowhead); data are also available in supplementary material as Movie 4. (C,D) Alexa568-Tf also enters the GFP-PIKfyve-positive compartment after 5 minutes, and was observed to exit the GFP-PIKfyve-positive early endosome through tubular elements. Series of frames (D) depicting Alexa568-transferrin-positive tubules exiting the GFP-PIKfyve early endsome; data are also available in supplementary material as Movie 5. Similar data were obtained in more than ten imaged cells for each case. Boxed areas in A and C indicate regions enlarged in the corresponding insets. Bars, 10 μm.
Fig. 4
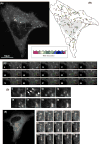
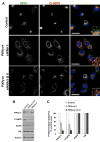
siRNA-mediated suppression of endogenous PIKfyve leads to formation of enlarged, swollen cytoplasmic vacuoles. Five siRNA duplexes (I–V) were designed, each targeting a distinct region of human PIKfyve (see supplementary material Table 1). (A) HeLa cells were transiently transfected with each individual siRNA or a combination of siRNA duplexes II and V, for 72 hours prior to determining the level of PIKfyve using an antibody against human PIKfyve. Tubulin was used as a loading control. Depending on the siRNA used, varying levels of suppression were achieved, with the highest suppression routinely obtained when using a combination of siRNAs II and V (ratio 1:1). Data are representative of one series of experiments typical of at least three other independent experiments. (B) HeLa cells were transiently transfected with either control siRNA, PIKfyve-specific siRNA duplex II or a combination of PIKfyve-specific siRNA duplexes II and V. After 72 hours, cells were fixed and analysed as phase-contrast images. Each image is of a random field of view. Arrowheads and arrows indicate small and large vacuoles, respectively. Bars, 45 μm. (C) Quantification of the percentage of cells displaying a vacuolar phenotype was determined by visual inspection. A cell was scored as having such a phenotype if two or more swollen vacuoles were visible. Data are from 100 cells imaged for each condition.
Fig. 5
PIKfyve suppression results in the swelling of both early and late endosomal compartments. (A–E) HeLa cells were transiently transfected with (A) PIKfyve-specific siRNA duplex II or (B–E) a combination of PIKfyve-specific siRNA duplexes II and V; left column, EM images; right column, phase-contrast images. Boxed insets show magnified regions. After 72 hours, cells were fixed and stained for (A) internalised transferrin receptor, 10 minutes after the addition of ligand (TfR), (B) LAMP1, (C) CD63, (D) SNX1 and (E) EEA1. For each condition similar images were observed in three independent experiments in which at least five randomly chosen fields of view were analysed. The arrows in (A) highlight the small vacuoles labelled with the internalised transferrin receptor. Bars, 10 μm (A), 25 μm (B–E).
Fig. 6
Suppression of PIKfyve does not affect endosomal sorting of the EGF receptor. HeLa cells were treated with either control, PIKfyve specific siRNA duplex II or a combination of PIKfyve specific siRNA duplexes II and V for 72 hours. (A) Cells were serum-starved for 3 hours, labelled with 125I-EGF at 1 kBq per well for 1 hour at 4°C, allowed to internalise surface-bound 125I-EGF for 5 minutes at 37°C and were then returned to 4°C. Cells were chased using medium containing 100 ng/ml unlabelled EGF for various times at 37°C. Recycled, degraded and internalised fractions were subjected to γ-radiation counting. (B) Serum-starved cells were stimulated with 100 ng/ml EGF for 0, 10, 20, 30, 60 and 120 minutes, and western blotted with antibodies against EGF receptor or tubulin (loading control). (C) Quantification of data from three independent experiments.
Fig. 7
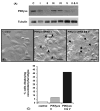
Suppression of PIKfyve alters the steady-state distribution of the CI-MPR and enhances receptor degradation. (A) HeLa cells were treated for 72 hours with control siRNA, PIKfyve-specific siRNA duplex II or a combination of siRNA duplexes II and V. Cells were fixed and stained against CI-MPR and EEA1 prior to imaging. Suppression of PIKfyve results in a redistribution of the CI-MPR from the TGN to peripheral punctae that show considerable overlap with the EEA1-labelled compartment. Boxed areas are magnified in right corner to show degree of overlap. Bars, 20 μm. (B) HeLa cells were treated for 72 hours with either control, PIKfyve-specific siRNA duplex II or a combination of PIKfyve-specific siRNA duplexes II and V. Cells were lysed and lysates blotted for PIKfyve, EGF receptor, transferrin and CI-MPR, and tubulin (loading control). Representative results are shown. (C) Quantification of data from four independent experiments.
Fig. 8
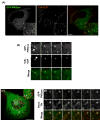
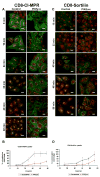
PIKfyve suppression alters the steady-state distribution of not only CI-MPR but also sortilin and furin. (A-C) HeLaM cells stably expressing either (A) CD8-CI-MPR, (B) CD8-sortilin or (C) CD8-furin, were treated with control siRNA or a combination of PIKfyve duplexes II and V as described in Materials and Methods. Cells were then fixed and co-stained for CD8 and EEA1 prior to confocal imaging. Images are projections of at least eight confocal sections and are representative of over 40 cells imaged for each condition. Bars, 40 μm.
Fig. 9
Kinetics of endosome-to-TGN retrieval of CI-MPR and sortilin is perturbed in PIKfyve-suppressed cells. (A,C) Images depicting the kinetics of delivery of a cell-surface-labelled (A) CD8-CI-MPR chimera or (C) CD8-sortillin chimera (both in green) to the TGN46-labelled TGN (red) in control HeLaM cells or in HeLaM cells whose PIKfyve was suppressed by a combination of duplex II and V. Images are projections of eight confocal sections and are representative of more than 40 cells images for each condition. Bars, 20 μm. (B,D) Quantification of the kinetics of cell-surface-labelled (B) CD8-CI-MPR chimera or (D) CD8-sortilin chimera transport to the TGN46-labelled TGN in HeLaM cells, expressed as percent normalised colocalisation between the two labels. Data were obtained as outlined in Materials and Methods from _n_≥40 cells for each time point, and are expressed as the mean ± s.e.m.
Fig. 10
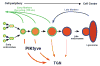
Model depicting the proposed role of PIKfyve in regulating endosome-to-TGN retrograde transport. Our model is adapted from the model proposed by Rink and colleagues, describing the process of early to late endosomal progression (Rink et al., 2005). In their model, early endosomes are viewed as forming a temporally dynamic network of compartments that through fusion and fission events are constantly generated and renewed in the cell periphery. Progression to late endosomes occurs through repeated fusion events that enrich degradative cargo (e.g. the EGF receptor shown in red) in increasingly fewer and larger endosomes that are localised in the centre of the cell. During this progression, early and late endosomes maintain their identity through the loss of early markers, such as transferrin receptor (depicted in green), and the acquisition of late markers shown in blue (Rink et al., 2005). Our data support a model whereby PIKfyve modulates endosome-to-TGN retrograde transport primarily, but perhaps not solely, from an early endosomal compartment. A loss of, or reduction in, the activity of PIKfyve leads to a decrease in this membrane flux, which in turn induces a progressive swelling of the endosomal compartments.
Similar articles
- Retrograde Transport from Early Endosomes to the trans-Golgi Network Enables Membrane Wrapping and Egress of Vaccinia Virus Virions.
Sivan G, Weisberg AS, Americo JL, Moss B. Sivan G, et al. J Virol. 2016 Sep 12;90(19):8891-905. doi: 10.1128/JVI.01114-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466413 Free PMC article. - PIKfyve regulation of endosome-linked pathways.
de Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbé S, Clague MJ. de Lartigue J, et al. Traffic. 2009 Jul;10(7):883-93. doi: 10.1111/j.1600-0854.2009.00915.x. Traffic. 2009. PMID: 19582903 Free PMC article. - Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport.
Carlton JG, Bujny MV, Peter BJ, Oorschot VM, Rutherford A, Arkell RS, Klumperman J, McMahon HT, Cullen PJ. Carlton JG, et al. J Cell Sci. 2005 Oct 1;118(Pt 19):4527-39. doi: 10.1242/jcs.02568. J Cell Sci. 2005. PMID: 16179610 Free PMC article. - Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.
Haeussler K, Ismaila AS, Malmenäs M, Noorduyn SG, Green N, Compton C, Thabane L, Vogelmeier CF, Halpin DMG. Haeussler K, et al. Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review. - Anti-VEGF drugs compared with laser photocoagulation for the treatment of diabetic retinopathy: a systematic review and meta-analysis.
Simmonds M, Llewellyn A, Walker R, Fulbright H, Walton M, Hodgson R, Bojke L, Stewart L, Dias S, Rush T, Lawrenson JG, Peto T, Steel D. Simmonds M, et al. Health Technol Assess. 2024 Dec;29(23):1-71. doi: 10.3310/PCGV5709. Health Technol Assess. 2024. PMID: 39673354
Cited by
- Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules.
van Weering JR, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ. van Weering JR, et al. EMBO J. 2012 Nov 28;31(23):4466-80. doi: 10.1038/emboj.2012.283. Epub 2012 Oct 19. EMBO J. 2012. PMID: 23085988 Free PMC article. - Metabolism and roles of phosphatidylinositol 3-phosphate in pollen development and pollen tube growth in Arabidopsis.
Gao XQ, Zhang XS. Gao XQ, et al. Plant Signal Behav. 2012 Feb;7(2):165-9. doi: 10.4161/psb.18743. Epub 2012 Feb 1. Plant Signal Behav. 2012. PMID: 22307045 Free PMC article. Review. - PIKfyve-ArPIKfyve-Sac3 core complex: contact sites and their consequence for Sac3 phosphatase activity and endocytic membrane homeostasis.
Ikonomov OC, Sbrissa D, Fenner H, Shisheva A. Ikonomov OC, et al. J Biol Chem. 2009 Dec 18;284(51):35794-806. doi: 10.1074/jbc.M109.037515. J Biol Chem. 2009. PMID: 19840946 Free PMC article. - Disruption of PIKFYVE causes congenital cataract in human and zebrafish.
Mei S, Wu Y, Wang Y, Cui Y, Zhang M, Zhang T, Huang X, Yu S, Yu T, Zhao J. Mei S, et al. Elife. 2022 Jan 13;11:e71256. doi: 10.7554/eLife.71256. Elife. 2022. PMID: 35023829 Free PMC article. - Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin.
Ikonomov OC, Fligger J, Sbrissa D, Dondapati R, Mlak K, Deeb R, Shisheva A. Ikonomov OC, et al. J Biol Chem. 2009 Feb 6;284(6):3750-61. doi: 10.1074/jbc.M806539200. Epub 2008 Dec 4. J Biol Chem. 2009. PMID: 19056739 Free PMC article.
References
- Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous