Progesterone receptor modulator for emergency contraception: a randomized controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
Progesterone receptor modulator for emergency contraception: a randomized controlled trial
Mitchell D Creinin et al. Obstet Gynecol. 2006 Nov.
Abstract
Objective: Compare the efficacy and adverse effects of CDB-2914, a new progesterone receptor modulator, to levonorgestrel for emergency contraception.
Methods: We performed a randomized, double-blinded noninferiority trial, enrolling healthy women seeking emergency contraception within 72 hours of unprotected intercourse. Participants were randomly assigned to receive a single dose of 50 mg of CDB-2914, plus a placebo 12 hours later or two doses of 0.75 mg of levonorgestrel taken 12 hours apart. Follow-up was scheduled 5 to 7 days after the expected onset of the next menstrual period. Posttreatment pregnancy was established by a positive urine test at follow-up and confirmed by quantitative serum beta-hCG. Daily diaries were used from the time of emergency contraception use until next menses to record adverse effects and sexual activity.
Results: Product efficacy was evaluable in 775 of CDB-2914 users and 774 of levonorgestrel users. Pregnancies occurred in 7 (0.9%, 95% confidence interval 0.2-1.6%) and 13 (1.7%, 95% confidence interval 0.8-2.6%) women, respectively. Based on the estimated cycle day of unprotected intercourse, 85% and 69% of anticipated pregnancies, respectively, were averted. Nausea was reported by a somewhat greater percentage of CDB-2914 than levonorgestrel users (29% compared with 24%, P=.03), but the distribution of other adverse effects was similar in both groups. Women in both groups experienced considerable variation in menstrual cycle length as compared with their reported individual normal cycle lengths.
Conclusion: CDB-2914 is at least as effective as levonorgestrel in preventing pregnancies after unprotected intercourse and has a similar side effect profile.
Level of evidence: I.
Trial registration: ClinicalTrials.gov NCT00271583.
Figures
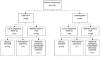
Fig. 1
Trial profile. Women for whom a pregnancy outcome could not be evaluated (lost to follow-up) were excluded from the modified intent-to-treat population. Women who were pregnant before treatment (based on serum hCG levels) or who used additional emergency contraception during the treatment cycle were excluded from the efficacy evaluable population. Creinin. CDB-2914 Emergency Contraception. Obstet Gynecol 2006.
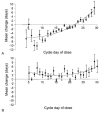
Fig. 2
Mean change in menstrual cycle length based on the cycle day of treatment. A. Levonorgestrel users. B. CDB-2914 users. Creinin. CDB-2914 Emergency Contraception. Obstet Gynecol 2006.
Comment in
- Emergency contraception: politics and science move forward.
Gilliam M. Gilliam M. Obstet Gynecol. 2006 Nov;108(5):1060-1. doi: 10.1097/01.AOG.0000245093.32653.d5. Obstet Gynecol. 2006. PMID: 17077224 No abstract available.
Similar articles
- Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis.
Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, Sogor L, Blithe DL, Scherrer B, Mathe H, Jaspart A, Ulmann A, Gainer E. Glasier AF, et al. Lancet. 2010 Feb 13;375(9714):555-62. doi: 10.1016/S0140-6736(10)60101-8. Epub 2010 Jan 29. Lancet. 2010. PMID: 20116841 Clinical Trial. - Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception.
Richardson AR, Maltz FN. Richardson AR, et al. Clin Ther. 2012 Jan;34(1):24-36. doi: 10.1016/j.clinthera.2011.11.012. Epub 2011 Dec 9. Clin Ther. 2012. PMID: 22154199 Review. - Ulipristal acetate for emergency contraception.
Snow SE, Melillo SN, Jarvis CI. Snow SE, et al. Ann Pharmacother. 2011 Jun;45(6):780-6. doi: 10.1345/aph.1P704. Epub 2011 Jun 10. Ann Pharmacother. 2011. PMID: 21666089 Review. - Ulipristal acetate as an emergency contraceptive agent.
Martinez AM, Thomas MA. Martinez AM, et al. Expert Opin Pharmacother. 2012 Sep;13(13):1937-42. doi: 10.1517/14656566.2012.705832. Epub 2012 Jul 7. Expert Opin Pharmacother. 2012. PMID: 22770536 Review. - Levonorgestrel versus the "Yuzpe" regimen. New choices in emergency contraception.
Lee SM, Dunn S, Evans MF. Lee SM, et al. Can Fam Physician. 1999 Mar;45:629-31. Can Fam Physician. 1999. PMID: 10099801 Free PMC article. Clinical Trial.
Cited by
- Association between levonorgestrel emergency contraception and the risk of ectopic pregnancy: a multicenter case-control study.
Zhang J, Li C, Zhao WH, Xi X, Cao SJ, Ping H, Qin GJ, Cheng L, Huang HF. Zhang J, et al. Sci Rep. 2015 Feb 12;5:8487. doi: 10.1038/srep08487. Sci Rep. 2015. PMID: 25674909 Free PMC article. - Emergency contraception. Widely available and effective but disappointing as a public health intervention: a review.
ESHRE CapriWorkshop Group. ESHRE CapriWorkshop Group. Hum Reprod. 2015 Apr;30(4):751-60. doi: 10.1093/humrep/dev019. Epub 2015 Feb 11. Hum Reprod. 2015. PMID: 25678571 Free PMC article. Review. - Levonorgestrel only emergency contraceptive use and risk of ectopic pregnancy in Eldoret Kenya: a case-control study.
Shurie S, Were E, Orang'o O, Keter A. Shurie S, et al. Pan Afr Med J. 2018 Nov 29;31:214. doi: 10.11604/pamj.2018.31.214.17484. eCollection 2018. Pan Afr Med J. 2018. PMID: 31447973 Free PMC article. - Ectopic pregnancy and emergency contraceptive pills: a systematic review.
Cleland K, Raymond E, Trussell J, Cheng L, Zhu H. Cleland K, et al. Obstet Gynecol. 2010 Jun;115(6):1263-1266. doi: 10.1097/AOG.0b013e3181dd22ef. Obstet Gynecol. 2010. PMID: 20502299 Free PMC article. Review. - Levonorgestrel emergency contraception and bodyweight: are current recommendations consistent with historic data?
Kardos L. Kardos L. J Drug Assess. 2020 Feb 10;9(1):37-42. doi: 10.1080/21556660.2020.1725524. eCollection 2020. J Drug Assess. 2020. PMID: 32166043 Free PMC article.
References
- Cheng L, Gulmezoglu AM, Oel CJ, Piaggio G, Ezcurra E, Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2004;(3):CD001324. - PubMed
- The Kaiser Family Foundation. Women's health care providers' experiences with emergency contraception. Available at: http://www.kff.org/womenshealth/3343-index.cfm. Retrieved June 23, 2003.
- Trussell J, Stewart F, Guest F, Hatcher RA. Emergency contraceptive pills: a simple proposal to reduce unintended pregnancies. Fam Plann Perspect. 1992;24:269–73. - PubMed
- Haspels AA. Emergency contraception: a review. Contraception. 1994;50:101–8. - PubMed
- Trussell J, Ellertson C, Stewart F, Raymond EG, Shochet T. The role of emergency contraception. Am J Obstet Gynecol. 2004;190:S30–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- N01-HD-9-3297/HD/NICHD NIH HHS/United States
- MO1RR000056/RR/NCRR NIH HHS/United States
- M01RR00096/RR/NCRR NIH HHS/United States
- N01-HD-6-3261/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials