Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver - PubMed (original) (raw)
Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver
Adam C Straub et al. Hepatology. 2007 Jan.
Abstract
Trivalent arsenic [As(III)] is a well-known environmental toxicant that causes a wide range of organ-specific diseases and cancers. In the human liver, As(III) promotes vascular remodeling, portal fibrosis, and hypertension, but the pathogenesis of these As(III)-induced vascular changes is unknown. To investigate the hypothesis that As(III) targets the hepatic endothelium to initiate pathogenic change, mice were exposed to 0 or 250 parts per billion (ppb) of As(III) in their drinking water for 5 weeks. Arsenic(III) exposure did not affect the overall health of the animals, the general structure of the liver, or hepatocyte morphology. There was no change in the total tissue arsenic levels, indicating that arsenic does not accumulate in the liver at this level of exposure. However, there was significant vascular remodeling with increased sinusoidal endothelial cell (SEC) capillarization, vascularization of the peribiliary vascular plexus (PBVP), and constriction of hepatic arterioles in As(III)-exposed mice. In addition to ultrastructural demonstration of SEC defenestration and capillarization, quantitative immunofluorescence analysis revealed increased sinusoidal PECAM-1 and laminin-1 protein expression, suggesting gain of adherens junctions and a basement membrane. Conversion of SECs to a capillarized, dedifferentiated endothelium was confirmed at the cellular level with demonstration of increased caveolin-1 expression and SEC caveolae, as well as increased membrane-bound Rac1-GTPase.
Conclusion: These data demonstrate that exposure to As(III) causes functional changes in SEC signaling for sinusoidal capillarization that may be initial events in pathogenic changes in the liver.
Conflict of interest statement
Potential conflict of interest: Dr. Stoltz and Dr. Barchowsky received grants from NIH. Dr. Straub received grants from University of Pittsburgh. Dr. Savay received grants from Dartmouth Medical School.
Figures
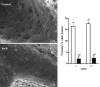
Fig. 1
Arsenic-stimulated capillarization of the liver sinusoidal endothelium. SEM images of sinusoidal vessels were obtained from thick sections of livers excised from control mice or mice exposed to 250 ppb As(III) for 5 weeks. In the graph, data are presented as mean ± SD porosity (open bars = control, closed bars = As(III)-exposed, n = 3), as determined in experimental procedures. Two-way ANOVA and Bonferroni’s post-test demonstrated no significant differences between zones, a highly significant effect of arsenic exposure relative to control (***P < 0.001).
Fig. 2
Arsenic-stimulated capillarization, basement membrane formation, and increased hepatocyte microvilli. (A) TEM images of sinusoidal vessels were captured from ultrathin sections of livers. Representative images are presented with portions magnified (B) to illustrate changes in the space of Disse and to show increased caveolae (arrows) in As(III)-exposed mice. (Bars = 500 nm) Abbreviations: SD, space of Disse; L, sinusoid lumen.
Fig. 3
Arsenic(III) induced expression of sinusoidal PECAM-1 and laminin protein. (A) Cryosections were immunostained for PECAM-1 (green) or laminin-1 (red). Merged images show DRAQ 5 stained nuclei (blue) (bar = 50 μm). (B) Images of portal vein and periportal PECAM-1 (green) and nuclear staining demonstrate PECAM changes in sinusoids, but not in the portal vein endothelium (L = lumen). (C) Quantitative morphometric analysis of midlobular PECAM-1 and laminin-1 protein staining is presented as the mean ± SD percentage of total positive-staining pixels for the respective protein per 400× microscopic field (**P < 0.01 and *P < 0.05, n = 5 mice).
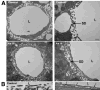
Fig. 4
As(III) stimulates vascularization of the PBVP and hepatic artery contraction. Serial sections were prepared from livers from control or As(III)-exposed mice. Sections were stained with either hematoxylin & eosin [(A) and (C)] or immunostained for PECAM-1 (green) and α-SMC actin (red) [(B) and (D)]. Final image magnification was 400× (BD = biliary ducts, HA = hepatic arteries). (E) The mean ± SD of PECAM-positive structures [* in images (B) and (D)] surrounding at least 2 biliary ducts per section from 5 mice is presented (***P < 0.001). (F) The luminal areas of hepatic arterioles were calculated. The data are the mean ± SD of the average luminal areas of at least 2 hepatic arterioles per section from 5 mice in each group. (**P < 0.01).
Fig. 5
Co-localization of As(III)-stimulated caveolin-1 and PECAM-1 protein expression. Sections of livers were immunostained for PECAM-1 (green) or caveolin-1 (red). Nuclei were stained with DRAQ 5 (blue). The white bar = 25 μm. The graph presents mean ± SD percentage of caveolin-1 positive pixels per 400× microscopic field (**P < 0.01; n = 5).
Fig. 6
Chronic As(III)-stimulated mobilization of Rac1 to SEC luminal membranes. Luminal SEC membranes of control and As(III) exposed mice were separated from total liver cell membranes (light fraction), as described in “Materials and Methods”. Rac1 and actin (loading control) abundance was measured by Western blotting. Each lane presents protein abundance in liver and SEC membrane fractions from individual mice.
Similar articles
- Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide.
Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, Pagano PJ, Stolz DB, Barchowsky A. Straub AC, et al. J Clin Invest. 2008 Dec;118(12):3980-9. doi: 10.1172/JCI35092. Epub 2008 Nov 13. J Clin Invest. 2008. PMID: 19033667 Free PMC article. - Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice.
Straub AC, Stolz DB, Vin H, Ross MA, Soucy NV, Klei LR, Barchowsky A. Straub AC, et al. Toxicol Appl Pharmacol. 2007 Aug 1;222(3):327-36. doi: 10.1016/j.taap.2006.10.011. Epub 2006 Oct 24. Toxicol Appl Pharmacol. 2007. PMID: 17123562 Free PMC article. - Arsenic requires sphingosine-1-phosphate type 1 receptors to induce angiogenic genes and endothelial cell remodeling.
Straub AC, Klei LR, Stolz DB, Barchowsky A. Straub AC, et al. Am J Pathol. 2009 May;174(5):1949-58. doi: 10.2353/ajpath.2009.081016. Epub 2009 Apr 6. Am J Pathol. 2009. PMID: 19349368 Free PMC article. - Alcohol-associated capillarization of sinusoids: A critique since the discovery by Schaffner and Popper in 1963.
Mak KM, Kee D, Shin DW. Mak KM, et al. Anat Rec (Hoboken). 2022 Jul;305(7):1592-1610. doi: 10.1002/ar.24829. Epub 2021 Nov 23. Anat Rec (Hoboken). 2022. PMID: 34766732 Review. - Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension?
Thabut D, Shah V. Thabut D, et al. J Hepatol. 2010 Nov;53(5):976-80. doi: 10.1016/j.jhep.2010.07.004. Epub 2010 Jul 24. J Hepatol. 2010. PMID: 20800926 Review.
Cited by
- Non-cirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and management.
Shukla A, Rockey DC, Kamath PS, Kleiner DE, Singh A, Vaidya A, Koshy A, Goel A, Dökmeci AK, Meena B, Philips CA, Sharma CB, Payawal DA, Kim DJ, Lo GH, Han G, Qureshi H, Wanless IR, Jia J, Sollano JD, Al Mahtab M, Muthiah MD, Sonderup MW, Nahum MS, Merican MIB, Ormeci N, Kawada N, Reddy R, Dhiman RK, Gani R, Hameed SS, Harindranath S, Jafri W, Qi X, Chawla YK, Furuichi Y, Zheng MH, Sarin SK. Shukla A, et al. Hepatol Int. 2024 Nov 15. doi: 10.1007/s12072-024-10739-6. Online ahead of print. Hepatol Int. 2024. PMID: 39546143 - Mechanisms of Metal-Induced Hepatic Inflammation.
Subramaniam NK, Mann KK. Subramaniam NK, et al. Curr Environ Health Rep. 2024 Nov 5. doi: 10.1007/s40572-024-00463-6. Online ahead of print. Curr Environ Health Rep. 2024. PMID: 39499483 Review. - Multiple roles of arsenic compounds in phase separation and membraneless organelles formation determine their therapeutic efficacy in tumors.
Qu M, He Q, Bao H, Ji X, Shen T, Barkat MQ, Wu X, Zeng LH. Qu M, et al. J Pharm Anal. 2024 Aug;14(8):100957. doi: 10.1016/j.jpha.2024.02.011. Epub 2024 Feb 24. J Pharm Anal. 2024. PMID: 39253293 Free PMC article. Review. - How single-cell transcriptomics provides insight on hepatic responses to TCDD.
Rance N. Rance N. Curr Opin Toxicol. 2023 Dec;36:100441. doi: 10.1016/j.cotox.2023.100441. Epub 2023 Sep 28. Curr Opin Toxicol. 2023. PMID: 37981901 - Hematological Abnormalities in Cirrhosis: A Narrative Review.
Lingas EC. Lingas EC. Cureus. 2023 May 19;15(5):e39239. doi: 10.7759/cureus.39239. eCollection 2023 May. Cureus. 2023. PMID: 37337504 Free PMC article. Review.
References
- Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–1049. - PubMed
- Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharmacol. 2005;206:169–175. - PubMed
- Guha Mazumder DN. Chronic arsenic toxicity: clinical features, epidemiology, and treatment: experience in West Bengal. J Environ Sci Health A. 2003;38:141–163. - PubMed
- Ng JC, Wang JP, Zheng B, Zhai C, Maddalena R, Liu F, et al. Urinary porphyrins as biomarkers for arsenic exposure among susceptible populations in Guizhou province, China. Toxicol Appl Pharmacol. 2005;206:176–184. - PubMed
- Shigeno K, Naito K, Sahara N, Kobayashi M, Nakamura S, Fujisawa S, et al. Arsenic trioxide therapy in relapsed or refractory japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. Int J Hematol. 2005;82:224–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES07373/ES/NIEHS NIH HHS/United States
- R01 CA076541/CA/NCI NIH HHS/United States
- P42 ES007373/ES/NIEHS NIH HHS/United States
- R29 CA076541/CA/NCI NIH HHS/United States
- CA76541/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous