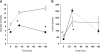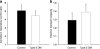Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle - PubMed (original) (raw)
Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle
R Boushel et al. Diabetologia. 2007 Apr.
Abstract
Aims/hypothesis: Insulin resistance and type 2 diabetes are associated with mitochondrial dysfunction. The aim of the present study was to test the hypothesis that oxidative phosphorylation and electron transport capacity are diminished in the skeletal muscle of type 2 diabetic subjects, as a result of a reduction in the mitochondrial content.
Materials and methods: The O(2) flux capacity of permeabilised muscle fibres from biopsies of the quadriceps in healthy subjects (n = 8; age 58 +/- 2 years [mean+/-SEM]; BMI 28 +/- 1 kg/m(2); fasting plasma glucose 5.4 +/- 0.2 mmol/l) and patients with type 2 diabetes (n = 11; age 62 +/- 2 years; BMI 32 +/- 2 kg/m(2); fasting plasma glucose 9.0 +/- 0.8 mmol/l) was measured by high-resolution respirometry.
Results: O(2) flux expressed per mg of muscle (fresh weight) during ADP-stimulated state 3 respiration was lower (p < 0.05) in patients with type 2 diabetes in the presence of complex I substrate (glutamate) (31 +/- 2 vs 43 +/- 3 pmol O(2) s(-1) mg(-1)) and in response to glutamate + succinate (parallel electron input from complexes I and II) (63 +/- 3 vs 85 +/- 6 pmol s(-1) mg(-1)). Further increases in O(2) flux capacity were observed in response to uncoupling by FCCP, but were again lower (p < 0.05) in type 2 diabetic patients than in healthy control subjects (86 +/- 4 vs 109 +/- 8 pmol s(-1) mg(-1)). However, when O(2) flux was normalised for mitochondrial DNA content or citrate synthase activity, there were no differences in oxidative phosphorylation or electron transport capacity between patients with type 2 diabetes and healthy control subjects.
Conclusions/interpretation: Mitochondrial function is normal in type 2 diabetes. Blunting of coupled and uncoupled respiration in type 2 diabetic patients can be attributed to lower mitochondrial content.
Figures
Fig. 1
Glucose (a) and insulin (b) concentrations in venous plasma before (t = 0 min) and during an OGTT. The patients with type 2 diabetes had higher fasting glucose levels and were severely insulin resistant compared with healthy control subjects (*p < 0.05). Black and white symbols represent healthy control subjects and patients with type 2 diabetes, respectively
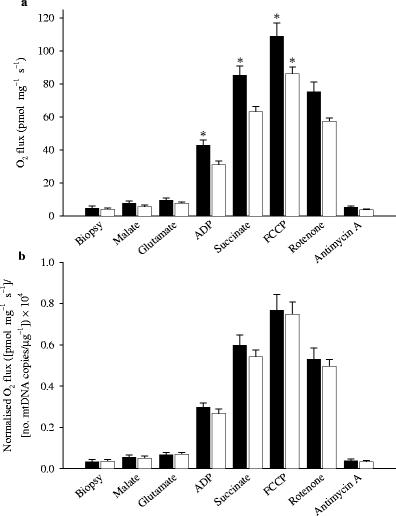
Fig. 2
O2 flux in permeabilised skeletal muscle fibres from patients with type 2 diabetes and healthy control subjects. Data are shown as O2 flux per mg of tissue (a) and further normalised to the number of copies of mtDNA per μg of tissue ×10,000 (b). When data are expressed relative to mtDNA, any difference between the groups disappears. Data are means±SEM (*p < 0.05). Black and white bars represent healthy control subjects and patients with type 2 diabetes, respectively
Fig. 3
a Respiratory control ratio for complex I (NADH supply from substrates glutamate + malate) measured as the ratio of O2 flux with (state 3) and without (state 2) ADP. b Electron transport capacity measured as O2 flux after FCCP-induced uncoupling relative to coupled O2 flux at state 3 with malate + glutamate + ADP + succinate (parallel electron input into both complex I and II). No significant difference between the groups was noted. Data are means±SEM
Similar articles
- Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes.
Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K. Mogensen M, et al. Diabetes. 2007 Jun;56(6):1592-9. doi: 10.2337/db06-0981. Epub 2007 Mar 9. Diabetes. 2007. PMID: 17351150 - Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes.
Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Toledo FG, et al. Diabetes. 2007 Aug;56(8):2142-7. doi: 10.2337/db07-0141. Epub 2007 May 29. Diabetes. 2007. PMID: 17536063 Clinical Trial. - Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology.
Gnaiger E. Gnaiger E. Int J Biochem Cell Biol. 2009 Oct;41(10):1837-45. doi: 10.1016/j.biocel.2009.03.013. Epub 2009 Apr 2. Int J Biochem Cell Biol. 2009. PMID: 19467914 Review. - Altered mitochondrial regulation in quadriceps muscles of patients with COPD.
Naimi AI, Bourbeau J, Perrault H, Baril J, Wright-Paradis C, Rossi A, Taivassalo T, Sheel AW, Rabøl R, Dela F, Boushel R. Naimi AI, et al. Clin Physiol Funct Imaging. 2011 Mar;31(2):124-31. doi: 10.1111/j.1475-097X.2010.00988.x. Epub 2010 Nov 22. Clin Physiol Funct Imaging. 2011. PMID: 21091605 - Mitochondrial function in skeletal muscle in type 2 diabetes.
Rabøl R. Rabøl R. Dan Med Bull. 2011 Apr;58(4):B4272. Dan Med Bull. 2011. PMID: 21466770 Review.
Cited by
- Inducible and reversible SOD2 knockdown in mouse skeletal muscle drives impaired pyruvate oxidation and reduced metabolic flexibility.
Ostrom EL, Stuppard R, Mattson-Hughes A, Marcinek DJ. Ostrom EL, et al. bioRxiv [Preprint]. 2024 Sep 25:2024.09.23.614547. doi: 10.1101/2024.09.23.614547. bioRxiv. 2024. PMID: 39386714 Free PMC article. Updated. Preprint. - Plant-derived compounds normalize platelet bioenergetics and function in hyperglycemia.
Gauer JS, Ajanel A, Kaselampao LM, Candir I, MacCannell ADV, Roberts LD, Campbell RA, Ariëns RAS. Gauer JS, et al. Res Pract Thromb Haemost. 2024 Aug 14;8(6):102548. doi: 10.1016/j.rpth.2024.102548. eCollection 2024 Aug. Res Pract Thromb Haemost. 2024. PMID: 39309231 Free PMC article. - Selection for Late Reproduction Leads to Loss of Complex I Mitochondrial Capacity and Associated Increased Longevity in Seed Beetles.
Mast HE, Blier PU, Ɖorđević M, Savković U, Holody CD, Bourque SL, Lemieux H. Mast HE, et al. J Gerontol A Biol Sci Med Sci. 2024 Nov 1;79(11):glae208. doi: 10.1093/gerona/glae208. J Gerontol A Biol Sci Med Sci. 2024. PMID: 39158488 Free PMC article. - Metabolic features of neutrophilic differentiation of HL-60 cells in hyperglycemic environments.
Cázares-Preciado JA, López-Arredondo A, Cruz-Cardenas JA, Luévano-Martínez LA, García-Rivas G, Prado-Garcia H, Brunck MEG. Cázares-Preciado JA, et al. BMJ Open Diabetes Res Care. 2024 Aug 8;12(4):e004181. doi: 10.1136/bmjdrc-2024-004181. BMJ Open Diabetes Res Care. 2024. PMID: 39122366 Free PMC article. - Exercise as a tool to mitigate metabolic disease.
Esteves JV, Stanford KI. Esteves JV, et al. Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C587-C598. doi: 10.1152/ajpcell.00144.2024. Epub 2024 Jul 9. Am J Physiol Cell Physiol. 2024. PMID: 38981607 Free PMC article. Review.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12351431', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12351431/'}\]}
- Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/BF01234508', 'is_inner': False, 'url': 'https://doi.org/10.1007/bf01234508'}, {'type': 'PubMed', 'value': '908476', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/908476/'}\]}
- Vondra K, Rath R, Bass A, Slabochová Z, Teisinger T, Vítek V (1977) Enzyme activities in quadriceps femoris muscle of obese diabetic male patients. Diabetologia 13:527–529 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11289047', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11289047/'}\]}
- He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50:817–823 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15894466', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15894466/'}\]}
- Ørtenblad N, Mogensen M, Petersen I et al (2005) Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 1741:206–214 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9216960', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9216960/'}\]}
- Simoneau JA, Kelley DE (1997) Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83:166–171 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical