Independently evolving species in asexual bdelloid rotifers - PubMed (original) (raw)
Independently evolving species in asexual bdelloid rotifers
Diego Fontaneto et al. PLoS Biol. 2007 Apr.
Abstract
Asexuals are an important test case for theories of why species exist. If asexual clades displayed the same pattern of discrete variation as sexual clades, this would challenge the traditional view that sex is necessary for diversification into species. However, critical evidence has been lacking: all putative examples have involved organisms with recent or ongoing histories of recombination and have relied on visual interpretation of patterns of genetic and phenotypic variation rather than on formal tests of alternative evolutionary scenarios. Here we show that a classic asexual clade, the bdelloid rotifers, has diversified into distinct evolutionary species. Intensive sampling of the genus Rotaria reveals the presence of well-separated genetic clusters indicative of independent evolution. Moreover, combined genetic and morphological analyses reveal divergent selection in feeding morphology, indicative of niche divergence. Some of the morphologically coherent groups experiencing divergent selection contain several genetic clusters, in common with findings of cryptic species in sexual organisms. Our results show that the main causes of speciation in sexual organisms, population isolation and divergent selection, have the same qualitative effects in an asexual clade. The study also demonstrates how combined molecular and morphological analyses can shed new light on the evolutionary nature of species.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
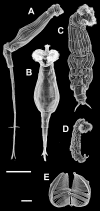
Figure 1. SEM Pictures of Some Species of the Genus Rotaria
(A) R. neptunia, lateral view; (B) R. macrura, ventral view; (C) R. tardigrada, dorsal view; (D) R. sordida, lateral view; and (E) trophi of R. tardigrada with open circles showing the location of landmarks used for the shape analysis. Scale bars: 100 μm for animals, 10 μm for trophi.
Figure 2. Scheme Showing the Predicted Patterns of Genetic and Morphological Variation Underlying Our Tests of Alternative Scenarios of Diversification
(A) Hypothetical trees showing expected genetic relationships among a sample of individuals under the null model that the sample is drawn from a single asexual population (H0) and under the alternative model that the clade has diversified into a set of independently evolving entities (H1). (B) Expected variation in two ecomorphological traits evolving either neutrally (H0) or by adaptive divergence (H1) in a genus that has diversified into six genetic clusters. Note that a mixed pattern is possible: Some genetic clusters may have experienced divergent selection on morphology, whereas others have not.
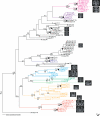
Figure 3. Phylogenetic Relationships in the Genus Rotaria
The consensus of 80,000 sampled trees from Bayesian analysis of the combined cox1 and 28S rDNA data sets is shown, displaying all compatible groupings and with average branch lengths proportional to numbers of substitutions per site under a separate GTR + invgamma substitution model for the cox1 and 28S partitions. Posterior probabilities above 0.5 and bootstrap support above 50% from a maximum parsimony bootstrap analysis are shown above and below each branch, respectively. Support values for within-species relationships are not shown for very short branches but are shown in Figures S1 through S3. Closed circles indicate clusters identified by the clustering analysis. Colors represent traditional species memberships. Diamonds indicate taxonomic species and monophyletic groups of Rotaria. Names refer to the species, the country, the number of site within that country for that species, and the number of individual from that site if several were isolated; for example, R.macr.IT.1.1 refers to the first individual from site 1 in Italy for R. macrura. Pictures of trophi from one individual from each cluster are shown to scale: Representatives of all sampled populations are shown in Figure S4. A full list of names and localities of samples is available in Table S1.
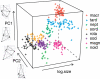
Figure 4. Plot of the Size and Shape (the First Two PCs, PC1 and PC2, from the Generalized Procrustes Analysis) of Trophi across Species
The directions of shape variation along each axis are shown for PC1 and PC2, respectively, using ×2 and ×4 magnification of the observed variation for emphasis. PC1 represents a continuum from oval to rounder trophi and from parallel to converging major teeth. PC2 represents a trend in the distance of the major teeth from the attachment point between the two halves of the trophi.
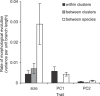
Figure 5. Evolutionary Rates of Changes in Trophi Size and Shape
Rates are expressed as the variance in each trait per unit branch length. Branch lengths are in units of the number of silent substitution per codon of cox1. Estimates from the maximum model with three rate classes are shown: within clusters, between clusters within taxonomic species, and between taxonomic species. Error bars show confidence limits within 2 log likelihood units of the maximum likelihood solution. Hierarchical likelihood ratio tests indicated that the model for size could be simplified to assume a joint rate for within cluster and between cluster branches (Table S5).
Comment in
- Who needs sex (or males) anyway?
Gross L. Gross L. PLoS Biol. 2007 Apr;5(4):e99. doi: 10.1371/journal.pbio.0050099. Epub 2007 Mar 20. PLoS Biol. 2007. PMID: 20076669 Free PMC article. No abstract available.
Similar articles
- Do Species Exist in Asexuals? Theory and Evidence from Bdelloid Rotifers.
Fontaneto D, Barraclough TG. Fontaneto D, et al. Integr Comp Biol. 2015 Aug;55(2):253-63. doi: 10.1093/icb/icv024. Epub 2015 Apr 24. Integr Comp Biol. 2015. PMID: 25912362 Review. - Sexual species are separated by larger genetic gaps than asexual species in rotifers.
Tang CQ, Obertegger U, Fontaneto D, Barraclough TG. Tang CQ, et al. Evolution. 2014 Oct;68(10):2901-16. doi: 10.1111/evo.12483. Epub 2014 Jul 25. Evolution. 2014. PMID: 24975991 Free PMC article. - Cryptic diversification in ancient asexuals: evidence from the bdelloid rotifer Philodina flaviceps.
Fontaneto D, Boschetti C, Ricci C. Fontaneto D, et al. J Evol Biol. 2008 Mar;21(2):580-7. doi: 10.1111/j.1420-9101.2007.01472.x. Epub 2007 Dec 13. J Evol Biol. 2008. PMID: 18081746 - Recombination in bdelloid rotifer genomes: asexuality, transfer and stress.
Wilson CG, Pieszko T, Nowell RW, Barraclough TG. Wilson CG, et al. Trends Genet. 2024 May;40(5):422-436. doi: 10.1016/j.tig.2024.02.001. Epub 2024 Mar 8. Trends Genet. 2024. PMID: 38458877 Review.
Cited by
- Patterns of molecular evolution in a parthenogenic terrestrial isopod (Trichoniscus pusillus).
Yarbrough E, Chandler C. Yarbrough E, et al. PeerJ. 2024 Jul 23;12:e17780. doi: 10.7717/peerj.17780. eCollection 2024. PeerJ. 2024. PMID: 39071119 Free PMC article. - Species Delimitation and Exploration of Species Partitions with ASAP and LIMES.
Puillandre N, Miralles A, Brouillet S, Fedosov A, Fischell F, Patmanidis S, Vences M. Puillandre N, et al. Methods Mol Biol. 2024;2744:313-334. doi: 10.1007/978-1-0716-3581-0_20. Methods Mol Biol. 2024. PMID: 38683328 - Circumtropical distribution and cryptic species of the meiofaunal enteropneust Meioglossus (Harrimaniidae, Hemichordata).
Defourneaux É, Herranz M, Armenteros M, Sørensen MV, Norenburg JL, Park T, Worsaae K. Defourneaux É, et al. Sci Rep. 2024 Apr 23;14(1):9296. doi: 10.1038/s41598-024-57591-0. Sci Rep. 2024. PMID: 38654022 Free PMC article. - Amazons Are Back: Absence of Males in a Praying Mantis from Uruguayan Savannas.
Trillo MC, Aisenberg A, Herberstein ME, Bidegaray-Batista L. Trillo MC, et al. Neotrop Entomol. 2024 Apr;53(2):323-329. doi: 10.1007/s13744-023-01114-5. Epub 2024 Feb 2. Neotrop Entomol. 2024. PMID: 38305945 - Sperm-dependent asexual species and their role in ecology and evolution.
Janko K, Mikulíček P, Hobza R, Schlupp I. Janko K, et al. Ecol Evol. 2023 Sep 28;13(10):e10522. doi: 10.1002/ece3.10522. eCollection 2023 Oct. Ecol Evol. 2023. PMID: 37780083 Free PMC article. Review.
References
- Coyne JA, Orr HA. Speciation. Sunderland (Massachusetts): Sinauer Associates; 2004. 545
- Hey J, Waples RS, Arnold ML, Butlin RK, Harrison RG. Understanding and confronting species uncertainty in biology and conservation. Trends Ecol Evol. 2003;18:597–603.
- Gavrilets S. Fitness landscapes and the origin of species. Princeton (New Jersey): Princeton University Press; 2004. 476
- Sites JW, Marshall JC. Delimiting species: A Renaissance issue in systematic biology. Trends Ecol Evol. 2003;18:462–470.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases