Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells - PubMed (original) (raw)
Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells
Takashi Koto et al. Am J Pathol. 2007 Apr.
Abstract
The mechanisms underlying the hypoxia-induced disruption of the barrier function of neural vasculature were analyzed with reference to the expression of claudin-5, a component of tight junctions between neural endothelial cells. The movement of claudin-5 from the cytoplasm to the plasma membrane of cultured confluent brain-derived endothelial (bEND.3) cells was closely correlated with the increase in the transendothelial electrical resistance. Inhibition of the expression of claudin-5 by RNAi resulted in a reduction of transendothelial electrical resistance, indicating a critical role of claudin-5 in the barrier property. Hypoxia (1% O(2)) altered the location of claudin-5 in the plasma membrane and the level of claudin-5 protein in bEND.3 cells, and these changes were accompanied by a decrease in the transendothelial electrical resistance. In vivo the claudin-5 molecules were expressed under normoxia in the plasma membrane of retinal microvascular endothelial cells but were significantly reduced under hypoxic conditions. Tracer experiments revealed that the barrier function of hypoxic retinal vasculature with depressed claudin-5 expression was selectively disrupted against small molecules, which is very similar to the phenotype of claudin-5-deficient mice. These in vitro and in vivo data indicate that claudin-5 is a target molecule of hypoxia leading to the disruption of the barrier function of neural vasculature.
Figures
Figure 1
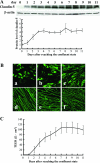
Claudin-5 expression and TEER in monolayer of bEND.3 cells. bEND.3 cells at the confluent state were cultured for an additional 11 days under normoxic condition, and the expression of claudin-5 (A, B) and barrier property of bEND.3 monolayer (C) was investigated. A: Western blotting with its quantitative analysis (bottom panel) showed that the cellular protein level of claudin-5 increased and reached a steady-state level around day 3, whereas the level of β-actin, a loading control, was almost unchanged. B: Immunocytochemistry showed that the predominant cytoplasmic location of claudin-5 immediately after reaching confluence (a) gradually changed to a relocation to the plasma membranes. Finally, claudin-5 was exclusively located to the plasma membranes 7 days after reaching confluence (d). a, 1 day; b, 3 days; c, 5 days; d, 7 days; e, 9 days; f, 11 days after reaching confluent state. C: The barrier property of bEND.3 monolayer was evaluated by measuring the TEER. A high correlation existed between the location of claudin-5 to the plasma membranes and the increase in the TEER of bEND.3 monolayer.
Figure 2
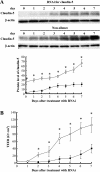
Relationship between the TEER of the bEND.3 monolayer and the expression levels of claudin-5. A: Expression level of claudin-5 in bEND.3 cells was altered by the RNAi technique, and Western blotting with quantitative analysis (bottom panel) confirmed the depressed claudin-5 expression in cells that were treated with siRNA specific for claudin-5 compared with the cells treated with non-silence oligonucleotides. The level of β-actin was used as a control for protein loading. B: bEND.3 cells with depressed expression of claudin-5 (closed square) failed to develop the monolayer with the TEER equivalent to that of bEND.3 monolayer without the depression of claudin-5 (open square). *P < 0.01.
Figure 3
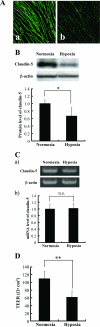
Hypoxia-induced changes in the expression of claudin-5. bEND.3 cells with claudin-5 located in the plasma membranes were cultured under either normoxia or hypoxia for 24 hours. Immunocytochemistry (A) and Western blotting (B) with its quantitative analysis (bottom panel in B) revealed that the localization of claudin-5 to plasma membranes [A (a, normoxia; b, hypoxia] as well as the cellular protein level of claudin-5 decreased under hypoxic condition. C: The mRNA level of claudin-5 was determined by RT-PCR (a) and real-time quantitative PCR (b). The claudin-5 mRNA level was approximately the same under normoxic and hypoxic conditions. Protein and mRNA levels of β-actin were also quantified as the controls of those of claudin-5. D: TEERs of bEND.3 monolayers under normoxia and hypoxia were measured, and the barrier property of bEND.3 cells was disrupted by hypoxia. *P < 0.05, **P < 0.01.
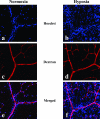
Figure 4
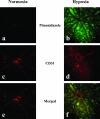
Oxygenation of retinas from mice under normoxia or hypoxia. To evaluate the tissue hypoxia, pimonidazole hydrochloride (Hypoxyprobe-1) was injected into the peritoneal cavities of mice maintained under either normoxic or hypoxic conditions for 7 days, and pimonidazole hydrochloride incorporated into hypoxic cells was detected by immunostaining (a, b). Immunostaining for CD31 was performed to examine the retinal vasculature (c, d). Merged view (e, f) figured the hypoxic metabolism of the retinas from mice that had been maintained under hypoxia, especially in the areas distant from the blood vessels.
Figure 5
In vivo effect of tissue hypoxia on the expression of claudin-5 in retinal blood vessels. Expression of claudin-5 in retinas from mice that had been maintained under normoxia or hypoxia for 7 days was investigated by immunofluorescence (A) and Western blotting (B). The level of β-actin was used as a loading control of Western blotting. A: Claudin-5 expression in the plasma membranes of retinal blood vessels is depressed in mice under hypoxia (b, d) in contrast to the distinct expression in the mice under normoxia (a, c). The decrease in claudin-5 expression by hypoxia was distinct in the peripheral blood vessels (arrows in a) compared with the reserved expression of claudin-5 in the proximal blood vessels (arrowheads in a) (c, d). B: Tissue protein level of claudin-5 significantly decreased in the hypoxic retina compared with the normoxic retina (top panel, gel of Western blotting; bottom panel, quantitative analysis of Western blotting for the relative decrease in claudin-5 expression in hypoxic retina). *P < 0.05.
Figure 6
Tracer experiment to evaluate the permeability of retinal blood vessels in vivo under normoxia or hypoxia. Injected tracers, Hoechst stain (a, b) and dextran (c, d), in normoxic (a, c, e) and hypoxic (b, d, f) retinas were detected under confocal microscopy. Merged views (e, f) of the signals of Hoechst stain and dextran are presented. Extensive nuclear staining by the extravasated Hoechst stain was noted in a hypoxic retina (f), whereas the stained nuclei were localized only in the vicinity of vascular lumen in a normoxic retina (e). On the other hand, no significant leakage of the injected dextran was detected in both normoxic (e) and hypoxic (f) retinas.
Similar articles
- Baicalin reduces the permeability of the blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells.
Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L, Lou J, Gao Y, Wang S, Wang Y. Zhu H, et al. J Ethnopharmacol. 2012 Jun 1;141(2):714-20. doi: 10.1016/j.jep.2011.08.063. Epub 2011 Sep 3. J Ethnopharmacol. 2012. PMID: 21920425 - Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells.
Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Ohtsuki S, et al. J Cell Physiol. 2007 Jan;210(1):81-6. doi: 10.1002/jcp.20823. J Cell Physiol. 2007. PMID: 16998798 - Microglia increase tight-junction permeability in coordination with Müller cells under hypoxic condition in an in vitro model of inner blood-retinal barrier.
Inada M, Xu H, Takeuchi M, Ito M, Chen M. Inada M, et al. Exp Eye Res. 2021 Apr;205:108490. doi: 10.1016/j.exer.2021.108490. Epub 2021 Feb 16. Exp Eye Res. 2021. PMID: 33607076 - Human cerebral microvessel endothelial cell culture as a model system to study the blood-brain interface in ischemic/hypoxic conditions.
Nagy Z, Vastag M, Kolev K, Bori Z, Karáidi I, Skopál J. Nagy Z, et al. Cell Mol Neurobiol. 2005 Feb;25(1):201-10. doi: 10.1007/s10571-004-1384-9. Cell Mol Neurobiol. 2005. PMID: 15962514 Review. - RNAi-mediated barrier modulation: synergies of the brain and eye.
Hanrahan F, Humphries P, Campbell M. Hanrahan F, et al. Ther Deliv. 2010 Oct;1(4):587-94. doi: 10.4155/tde.10.49. Ther Deliv. 2010. PMID: 22833969 Review.
Cited by
- Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow.
Pan Q, He C, Liu H, Liao X, Dai B, Chen Y, Yang Y, Zhao B, Bihl J, Ma X. Pan Q, et al. Mol Brain. 2016 Jun 7;9(1):63. doi: 10.1186/s13041-016-0243-1. Mol Brain. 2016. PMID: 27267759 Free PMC article. - Nonlytic exocytosis of Cryptococcus neoformans from neutrophils in the brain vasculature.
Yang X, Wang H, Hu F, Chen X, Zhang M. Yang X, et al. Cell Commun Signal. 2019 Sep 9;17(1):117. doi: 10.1186/s12964-019-0429-0. Cell Commun Signal. 2019. PMID: 31500648 Free PMC article. - Novel characterization of bEnd.3 cells that express lymphatic vessel endothelial hyaluronan receptor-1.
Yuen D, Leu R, Tse J, Wang S, Chen LL, Chen L. Yuen D, et al. Lymphology. 2014 Jun;47(2):73-81. Lymphology. 2014. PMID: 25282873 Free PMC article. - Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells.
Kluger MS, Clark PR, Tellides G, Gerke V, Pober JS. Kluger MS, et al. Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):489-500. doi: 10.1161/ATVBAHA.112.300893. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288152 Free PMC article. - Imatinib enhances functional outcome after spinal cord injury.
Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, Eriksson U. Abrams MB, et al. PLoS One. 2012;7(6):e38760. doi: 10.1371/journal.pone.0038760. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22723886 Free PMC article.
References
- Ikeda E, Flamme I, Risau W. Developing brain cells produce factors capable of inducing the HT7 antigen, a blood-brain barrier-specific molecule, in chick endothelial cells. Neurosci Lett. 1996;209:149–152. - PubMed
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. - PubMed
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. - PubMed
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. - PubMed
- Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25(Suppl):523–550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources