Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination - PubMed (original) (raw)
Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination
Helen Horton et al. J Immunol Methods. 2007.
Abstract
Candidate HIV-1 vaccines currently being evaluated in clinical trials are designed to elicit HIV-1-specific cellular immunity. Intracellular cytokine staining (ICS) assays allow sensitive, quantitative ex vivo assessments of antigen-specific T cells including immunophenotyping of responding cells and measurement of multiple effector functions. Additionally, the use of banked cryopreserved PBMC samples makes this assay attractive in the setting of large efficacy trials where it is less feasible to perform immunoassays on freshly isolated samples. Here we describe extensive studies to optimize and quantitatively validate the 8-color ICS assay for use in clinical trials of candidate vaccines, which includes measurement of viable IFN-gamma, IL-2, TNF-alpha and IL-4 producing CD4+ and CD8+ T cells. We show that omission of viability dye staining results in an over-estimate of the true antigen-specific T cell response by up to two-fold. After optimization, the 8-color assay was validated for specificity, precision, linearity, limit of quantitation and robustness. The assay has a lower quantitation limit generally below 0.04%, depending on the cytokine subset. Additionally, with appropriate gating, the 8-color assay gives comparable cytokine-positive responses to those observed with the conventional 4-color assay. In conclusion, we provide the first description of a quantitatively validated ICS assay, which permits quantitative and qualitative evaluation of vaccine-induced immunogenicity and analysis of immune correlates of protection.
Figures
Figure 1
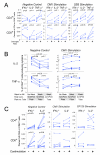
A. Culture of cells overnight after thaw increases cytokine responses. Cryopreserved PBMC from four healthy individuals were thawed, washed, resuspended at 5 × 106 cells/ml and added to the wells of two 96-well round-bottom plates. For one plate, the cells were stimulated immediately for six hours with DMSO, CMV or SEB, including co-stimulation and Brefeldin A. Cells were then processed by ICS. The other plate was placed into culture overnight (rested), and then stimulated for six hours followed by ICS. Three 4-color staining panels were used that included CD3, CD4, CD8 and the cytokine (IFN-γ, IL-2 or TFN-α. Panel A in Table 1). The upper panels show the CD4+ T cell responses, and the lower panels show the CD8+ T cell responses. The left panels show responses to co-stimulation only (negative control), the middle panels show responses to the CMVpp65 peptide pool, and the right panels show the response to SEB. Each color represents a different subject, and the lines join responses without or with overnight rest. All data for CMV and SEB are background subtracted. Paired T test p values are shown on the graphs. B. Washing cells after overnight rest decreases background for IL-2 and TNF-α, but does not decrease CMV-specific responses. Three variables were examined in this experiment: resting concentration, incubation vessel for rest and stimulation, and washing vs. not washing cells in the morning after the overnight rest. For the first condition (left column in each graph), cells were rested at 5 × 106/ml in a 96-well round-bottom plate and were not washed before stimulation the next morning. The second condition (middle column in each graph) was the same except that the cells were washed in the morning before stimulation. For the third condition, cells were rested at 2 × 106/ml in 50 ml conical tubes and were washed in the morning before stimulation. CD4+ T cell responses to IL-2 (upper panels) and TNF-α (lower panels) are shown. The left panels show responses for the negative control, and the right panels show the responses to CMV. Six different PBMC samples were tested, and data for each sample are connected by lines. The 8-color ICS panel was used. Paired T test p values for comparisons between each of the three conditions are shown on the graphs. C. Co-stimulation with α—CD28/CD49d increases background responses, but does not increase antigen-specific responses after background is subtracted. PBMC samples from five participants in HVTN protocol 041 (subunit protein vaccine including Gp120 and a Nef/Tat fusion protein) (Goepfert et al., 2005) were stimulated with a gp120 peptide pool (right panels), with the CMVpp65 peptide pool (middle panels), or with DMSO (negative control, left panels). The percentages of CD4+ (upper) and CD8+ (lower) T cells producing IFN-γ and IL-2 are shown. Within each graph, responses with or without co-stimulation are compared. Lines connect responses from the same PBMC donor. Gp120 and CMV results are shown with background subtracted. For this experiment, cells were rested overnight at 5 × 106/ml and were washed before stimulation. Paired T test p values are shown for the negative control. Comparisons for the Gp120 and CMV are not significant.
Figure 2
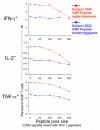
Cytokine-producing T cells recognizing an individual CMV pp65 15 amino acid peptide decrease as the total pool size increases. PBMC from two subjects with previously documented responses to individual peptides were examined [subject 1584 (red) responded to CMV peptide AGILARNLVPMVATV, and subject 2622 (blue) responded to CMV peptide TERKTPRVTGGGAMA]. Each of these peptides was mixed with increasing numbers of HIV-1 Gag and Pol peptides, as listed on the abscissa; and the IFN-γ, IL-2 and TNF-α CD8+ T cell responses were measured by 4-color ICS. The response to the peptide alone is shown as the first data point in each graph. One outlier for the IFN-γ response (10% response) to peptide TERKTPRVTGGGAMA in the 200 peptide pool size in subject 262 was removed, as indicated by the asterisk.
Figure 3
Staining profile for 8-color ICS. A. Shown is PBMC sample stimulated with SEB. The top row of plots shows sequentially, left to right, the gating hierarchy for PBMC, singlets (single cells), lymphocytes, live cells, CD3+ T cells, and CD4+ or CD8+ T cells. The next two rows show the gates used to identify cytokine-producing CD4+ (middle row) and CD8+ T cells (lower row). Each cytokine is gated individually. The co-expression of all the cytokines is determined in the software analysis by using Boolean gates based on these single-positive gates. B. Shown is the co-expression of IFN-γ and IL-2 for CD4+ and CD8+ T cells for the same PBMC sample shown in A after stimulation with SEB. C. Shown is the co-expression of IFN-γ and IL-2 for CD4+ and CD8+ T cells for a PBMC sample after stimulation with the CMV pp65 peptide pool. The percentage of either CD4+ or CD8+ T cells is indicated in each quadrant.
Figure 4
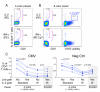
Dead cells can cause non-specific cytokine staining, especially for PE-conjugated reagents. A. Shown are the IL-2 (upper) and IFN-γ (lower) CD4+ T cell responses to CMV in one PBMC sample. The expression of CD8 is shown on the abscissa to display the different staining pattern of CD8 on the dim cytokine-staining vs. the bright cytokine-staining cells. The percent of CD4+ T cells in an open cytokine gate or a high gate is shown. Four-color staining panels including CD3, CD4, CD8 and the cytokine on PE were used (the first staining panel in table 1 with the PE IFN-g omitted, analyzed on the LSRII). B. Shown are the responses to CMV stimulation for the same PBMC sample as shown in A, determined by the 8-color ICS panel. The left panels show the expression of the cytokine vs. CD8. The middle panels show the expression of the cytokine vs. the viability marker. C. The percentage of CD4+ T cells expressing IL-2 is shown for seven PBMC samples stimulated with CMV (left) or with DMSO (negative control, right). Within each graph, the results for the 8-color panel using a high cytokine gate are shown. The other three columns show the results for staining with a modified 4-color panel that includes the violet viability marker (the third staining panel in table 1), and compares 1) the frequency of IL-2-producing CD4+ T cells when dead cells are not gated out and a standard open cytokine gate is used (left column); 2) when dead cells are gated out and the standard cytokine gate is used (next column); or 3) when dead cells are not gated out and the high cytokine gate is used (third column). Cells have been gated as CD3+, and CD4+CD8-.
Figure 5
Summary of the ICS protocol is shown. This diagram outlines the major steps in the ICS protocol used for analysis of trial samples. The protocol requires three days. Plates frozen on day 2 can be kept at −80deg for at least four weeks before proceeding to the next steps as shown on day 3.
Figure 6
A. Quantitative validation of the 8-color ICS assay demonstrates that the assay is linear in the detection of IFN-γ alone and IFN-γ. in combination with IL-2 for CD4 and CD8+ T cells. Shown are data from one representative experiment where CMV-stimulated PBMC are diluted into un-stimulated autologous PBMC. Three donors were used for these experiments, chosen because they had three different levels of total cytokine response to CMV. The donor with the medium response is shown here. At each cell dilution, the assay was performed in triplicate. Cells producing both IF -γ and IL-2 (left panels) or only IFN-γ (right panels) for either CD4+ T cells (upper) or CD8+ T cells (lower) are shown. The Y-axis shows the percentage of CD4+ or CD8+ T cells producing the indicated cytokine(s). The cell dilution is shown on the X-axis as the percentage of undiluted CMV-stimulated cells in the dilution. The intra-sample variability in this one experiment is visualized by the range of responses at each dilution level. The standard least squares correlation is listed. B. The CV for measurement of cytokine responses increases as the level of the cytokine response decreases and the limit of quantitation is determined as the lowest cytokine response for which the CV remains ≤30%. Shown is one of the experiments used to determine limit of quantitation. These are the same experiments used to determine linearity, and the four cytokine subsets are shown as in Figure 6A. Data shown here are from the PBMC donor with the high CMV response. The triplicates at each dilution level are averaged (shown in green), and the scale for this mean response is shown on the right vertical axis. The red line denotes the intra-sample CV at each dilution level for each cytokine subset. The scale for the CV is shown on the left vertical axis. The dashed red line indicates the acceptable upper limit of CV, 30%. The arrow points to the lowest mean cytokine response for which the CV is below 30%. C. The limit of quantitation is calculated for each type of variation for each PBMC sample and for each cytokine subset. Cells producing both IF
-γ and IL-2 (left panels) or only IFN-γ (right panels) for either CD4+ T cells (upper) or CD8+ T cells (lower) are shown. The Y-axis shows the percentage of CD4+ or CD8+ T cells producing the indicated cytokine(s). The cell dilution is shown on the X-axis as the percentage of undiluted CMV-stimulated cells in the dilution. The intra-sample variability in this one experiment is visualized by the range of responses at each dilution level. The standard least squares correlation is listed. B. The CV for measurement of cytokine responses increases as the level of the cytokine response decreases and the limit of quantitation is determined as the lowest cytokine response for which the CV remains ≤30%. Shown is one of the experiments used to determine limit of quantitation. These are the same experiments used to determine linearity, and the four cytokine subsets are shown as in Figure 6A. Data shown here are from the PBMC donor with the high CMV response. The triplicates at each dilution level are averaged (shown in green), and the scale for this mean response is shown on the right vertical axis. The red line denotes the intra-sample CV at each dilution level for each cytokine subset. The scale for the CV is shown on the left vertical axis. The dashed red line indicates the acceptable upper limit of CV, 30%. The arrow points to the lowest mean cytokine response for which the CV is below 30%. C. The limit of quantitation is calculated for each type of variation for each PBMC sample and for each cytokine subset. Cells producing both IF -γ and IL-2 (left panels) or only IFN-γ (right panels) for either CD4+ T cells (upper) or CD8+ T cells (lower) are shown. The type of variation is shown on the X-axis grouped by the three PBMC donors with low, medium or high CMV responses. For the medium responder, an additional type of variation, inter-sample, was evaluated. The limit of quantitation is shown on the Y-axis as the percent of CD4+ or CD8+ T cells. This was determined as in Figure 6B, i.e., the lowest cytokine response for which the CV remains ≤30%. One high outlier (0.6%, marked as *) was removed for the intra-sample analysis of the medium donor for the CD8+ IFN-γ+IL-2- T cell response.
-γ and IL-2 (left panels) or only IFN-γ (right panels) for either CD4+ T cells (upper) or CD8+ T cells (lower) are shown. The type of variation is shown on the X-axis grouped by the three PBMC donors with low, medium or high CMV responses. For the medium responder, an additional type of variation, inter-sample, was evaluated. The limit of quantitation is shown on the Y-axis as the percent of CD4+ or CD8+ T cells. This was determined as in Figure 6B, i.e., the lowest cytokine response for which the CV remains ≤30%. One high outlier (0.6%, marked as *) was removed for the intra-sample analysis of the medium donor for the CD8+ IFN-γ+IL-2- T cell response.
Similar articles
- Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein-Barr virus-specific T cells as targets of interest in immunotherapeutic approaches.
Tischer S, Dieks D, Sukdolak C, Bunse C, Figueiredo C, Immenschuh S, Borchers S, Stripecke R, Maecker-Kolhoff B, Blasczyk R, Eiz-Vesper B. Tischer S, et al. J Immunol Methods. 2014 Jun;408:101-13. doi: 10.1016/j.jim.2014.05.011. Epub 2014 May 28. J Immunol Methods. 2014. PMID: 24877879 - Optimization and qualification of an 8-color intracellular cytokine staining assay for quantifying T cell responses in rhesus macaques for pre-clinical vaccine studies.
Donaldson MM, Kao SF, Eslamizar L, Gee C, Koopman G, Lifton M, Schmitz JE, Sylwester AW, Wilson A, Hawkins N, Self SG, Roederer M, Foulds KE. Donaldson MM, et al. J Immunol Methods. 2012 Dec 14;386(1-2):10-21. doi: 10.1016/j.jim.2012.08.011. Epub 2012 Aug 28. J Immunol Methods. 2012. PMID: 22955212 Free PMC article. - Vaccination in humans generates broad T cell cytokine responses.
De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. De Rosa SC, et al. J Immunol. 2004 Nov 1;173(9):5372-80. doi: 10.4049/jimmunol.173.9.5372. J Immunol. 2004. PMID: 15494483 - Vaccine applications of flow cytometry.
De Rosa SC. De Rosa SC. Methods. 2012 Jul;57(3):383-91. doi: 10.1016/j.ymeth.2012.01.001. Epub 2012 Jan 10. Methods. 2012. PMID: 22251671 Free PMC article. Review. - Immunological monitoring of cancer vaccine therapy.
Nagorsen D, Scheibenbogen C, Thiel E, Keilholz U. Nagorsen D, et al. Expert Opin Biol Ther. 2004 Oct;4(10):1677-84. doi: 10.1517/14712598.4.10.1677. Expert Opin Biol Ther. 2004. PMID: 15461579 Review.
Cited by
- A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants.
Tameris M, Hokey DA, Nduba V, Sacarlal J, Laher F, Kiringa G, Gondo K, Lazarus EM, Gray GE, Nachman S, Mahomed H, Downing K, Abel B, Scriba TJ, McClain JB, Pau MG, Hendriks J, Dheenadhayalan V, Ishmukhamedov S, Luabeya AK, Geldenhuys H, Shepherd B, Blatner G, Cardenas V, Walker R, Hanekom WA, Sadoff J, Douoguih M, Barker L, Hatherill M. Tameris M, et al. Vaccine. 2015 Jun 9;33(25):2944-54. doi: 10.1016/j.vaccine.2015.03.070. Epub 2015 Apr 28. Vaccine. 2015. PMID: 25936724 Free PMC article. Clinical Trial. - Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001).
Barouch DH, Liu J, Peter L, Abbink P, Iampietro MJ, Cheung A, Alter G, Chung A, Dugast AS, Frahm N, McElrath MJ, Wenschuh H, Reimer U, Seaman MS, Pau MG, Weijtens M, Goudsmit J, Walsh SR, Dolin R, Baden LR. Barouch DH, et al. J Infect Dis. 2013 Jan 15;207(2):248-56. doi: 10.1093/infdis/jis671. Epub 2012 Nov 2. J Infect Dis. 2013. PMID: 23125443 Free PMC article. Clinical Trial. - Harmonization and qualification of intracellular cytokine staining to measure influenza-specific CD4+ T cell immunity within the FLUCOP consortium.
Begue S, Waerlop G, Salaun B, Janssens M, Bellamy D, Cox RJ, Davies R, Gianchecchi E, Medaglini D, Montomoli E, Pettini E, Leroux-Roels G, Clement F, Pagnon A. Begue S, et al. Front Immunol. 2022 Oct 20;13:982887. doi: 10.3389/fimmu.2022.982887. eCollection 2022. Front Immunol. 2022. PMID: 36341380 Free PMC article. - Cellular and humoral responses to an HIV DNA prime by electroporation boosted with recombinant vesicular stomatitis virus expressing HIV subtype C Env in a randomized controlled clinical trial.
Wilson GJ, Rodriguez B, Li SS, Allen M, Frank I, Rudnicki E, Trahey M, Kalams S, Hannaman D, Clarke DK, Xu R, Egan M, Eldridge J, Pensiero M, Latham T, Ferrari G, Montefiori DC, Tomaras GD, De Rosa SC, Jacobson JM, Miner MD, Elizaga M; HIV Vaccine Trials Network 112 Protocol Team. Wilson GJ, et al. Vaccine. 2023 Apr 17;41(16):2696-2706. doi: 10.1016/j.vaccine.2023.03.015. Epub 2023 Mar 17. Vaccine. 2023. PMID: 36935288 Free PMC article. Clinical Trial. - A robust pipeline for high-content, high-throughput immunophenotyping reveals age- and genetics-dependent changes in blood leukocytes.
Liechti T, Van Gassen S, Beddall M, Ballard R, Iftikhar Y, Du R, Venkataraman T, Novak D, Mangino M, Perfetto S, Larman HB, Spector T, Saeys Y, Roederer M. Liechti T, et al. Cell Rep Methods. 2023 Oct 23;3(10):100619. doi: 10.1016/j.crmeth.2023.100619. Cell Rep Methods. 2023. PMID: 37883924 Free PMC article.
References
- Bach MK, Brashler JR. Isolation of subpopulations of lymphocytic cells by the use of isotonically balanced solutions of Ficoll. I. Development of methods and demonstration of the existence of a large but finite number of subpopulations. Exp Cell Res. 1970;61:387–96. - PubMed
- Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol Suppl. 1976;5:9–15. - PubMed
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–6. - PubMed
- Cohen J. Public health. AIDS vaccine trial produces disappointment and confusion. Science. 2003;299:1290–1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027757-21/AI/NIAID NIH HHS/United States
- U01 AI068618-03/AI/NIAID NIH HHS/United States
- P30 AI 27757/AI/NIAID NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- U01 AI068618/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials