CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells - PubMed (original) (raw)
CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells
E Kabingu et al. Br J Cancer. 2007.
Abstract
Cancer survival rates decrease in the presence of disseminated disease. However, there are few therapies that are effective at eliminating the primary tumour while providing control of distant stage disease. Photodynamic therapy (PDT) is an FDA-approved modality that rapidly eliminates local tumours, resulting in cure of early disease and palliation of advanced disease. Numerous pre-clinical studies have shown that local PDT treatment of tumours enhances anti-tumour immunity. We hypothesised that enhancement of a systemic anti-tumour immune response might control the growth of tumours present outside the treatment field. To test this hypothesis we delivered PDT to subcutaneous (s.c.) tumours of mice bearing both s.c. and lung tumours and monitored the growth of the untreated lung tumours. Our results demonstrate that PDT of murine tumours provided durable inhibition of the growth of untreated lung tumours. The inhibition of the growth of tumours outside the treatment field was tumour-specific and dependent on the presence of CD8(+) T cells. This inhibition was accompanied by an increase in splenic anti-tumour cytolytic activity and by an increase in CD8(+) T cell infiltration into untreated tumours. Local PDT treatment led to enhanced anti-tumour immune memory that was evident 40 days after tumour treatment and was independent of CD4(+) T cells. CD8(+) T cell control of the growth of lung tumours present outside the treatment field following PDT was dependent upon the presence of natural killer (NK) cells. These results suggest that local PDT treatment of tumours lead to induction of an anti-tumour immune response capable of controlling the growth of tumours outside the treatment field and indicate that this modality has potential in the treatment of distant stage disease.
Figures
Figure 1
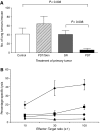
Control of distant disease by PDT is immune cell mediated. (A) S.c. tumours present on BALB/cJ mice bearing s.c. and lung tumours were either treated with light only (Control, _n_=5), surgically removed 18–24 h post-Photofrin administration (SR, _n_=16), or received PDT (_n_=16). The PDT/skin column (_n_=6) represents results from mice bearing both s.c. and lung tumours that were treated with Photofrin and had a 1 cm3 area of skin on the non-tumour bearing shoulder illuminated with 630 nm light. The average number of lung tumours was determined as described in Materials and Methods; error bars represent s.e.m. Significance was calculated by Student's _t_-test. (B) Spleen cells isolated from naïve animals (•) or from mice whose s.c. tumours were surgically removed (▪) were mixed with 51Cr-labeled EMT6 target cells at various effector-to-target cell ratios. Spleen cells from mice whose tumours were PDT treated were mixed with either 51Cr-labeled EMT6 (□) or Colon 26 (▴) target cells. Effector and target cells were incubated for 4 h and the percent specific lysis was determined as described in Materials and Methods. Spleen cells from a minimum of six mice were used for each point; error bars represent s.e.m.
Figure 2
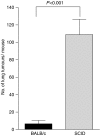
Control of distant tumours by PDT is dependent upon the presence of adaptive immune cells. S.c. tumours present on BALB/cJ or SCID mice bearing s.c. and lung tumours were treated with PDT (BALB/cJ, _n_=16; SCID, _n_=18). The average number of lung tumours was determined as described in Materials and Methods; error bars represent s.e.m. Significance was calculated by Student's _t_-test.
Figure 3
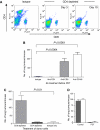
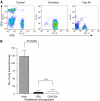
Control of distant disease by PDT is mediated by CD8+ T cells. (A) BALB/cJ mice were treated with isotype control, anti-CD4 or anti-CD8 monoclonal antibodies. Antibody treated animals were bled and the extent of the depletion was confirmed by flow cytometry. Representative dot plots are shown of samples collected on day 3 and day 10. (B) BALB/cJ mice were treated with isotype control (_n_=11), anti-CD4 (_n_=11), anti-CD8 (_n_=10), or a combination of anti-CD4 and anti-CD8 antibodies (_n_=11) before inoculation with EMT6 tumour cells. S.c. EMT6 tumours were treated with Photofrin-PDT (135 J cm−2 given at 75 mW cm−2) and 10 days post-PDT the average number of lung tumours was determined. (C) Splenocytes were harvested from mice depleted of immune cells as in (B) 3 days after PDT treatment of s.c. tumours and adoptively transferred into SCID mice (107 cells mouse−1). Recipient animals were challenged with 104 EMT6 tumour cells 2 days post-transfer; the presence of lung tumours was assessed 10 days after tumour challenge. (D) S.c. EMT6 tumours of BALB/cJ (filled bars) or CD40-/- (open bars) mice bearing s.c. and lung tumours were treated with Photofrin-PDT. Ten days post-PDT the average number of lung tumours was determined as described in Materials and Methods. Each group contains a minimum of five mice. Error bars represent s.e.m. in all figures; significance was calculated by Student's _t_-test.
Figure 4
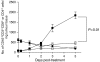
Increased CD8+ T-cell infiltrate into distant EMT6 tumours following PDT. The left tumour of mice bearing a tumour on each shoulder was treated with PDT or removed surgically (SR); at various time post-treatment the right untreated tumours were removed and examined by flow cytometry for the presence of CD45+, CD3+, CD4+ cells (circles) or CD45+, CD3+, CD8+ cells (squares) as described previously (Gollnick et al, 1997). Results are presented as the number of cells per mg of tumour tissue. Open symbols represent the infiltrate present in untreated tumours when the left tumour was removed surgically; closed symbols represent the infiltrate present in right tumours following treatment of the left tumour with PDT. Tumours from a minimum of three animals were examined from each group. Error bars represent s.e.m.; significance was calculated by Student's _t_-test.
Figure 5
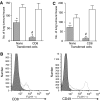
Enhancement of memory CD8+ T cells by PDT is independent of CD4+ T cells. Spleen cells were isolated from naïve BALB/cJ mice and CD8+ or CD 4+ T cells were enriched by negative selection as described in Materials and Methods. (A) An aliquot of the enriched population was subjected to flow cytometry to assess purity. A representative dot plot from the starting population (Control), the enriched CD8+ T-cell population and a recipient mouse after tumour challenge (day 40) is shown. (B) The enriched CD8+ T cell or combined CD8+ and CD4+ T cell populations was adoptively transferred into SCID mice (107 mouse−1). Recipient mice were inoculated with EMT6 cells and the resulting tumours were treated with Photofrin-PDT. Mice cured of tumours were challenged >40 days post-treatment by i.v. injection of EMT6 cells; 10 days following challenge lungs were examined for the presence of tumours The average number of lung tumours was determined as described in Materials and Methods; error bars represent s.e.m. Significance was calculated by Student's _t_-test.
Figure 6
NK cells contribute to control of distant tumours by local PDT. (A) SCID mice were treated with TM_β_1 to deplete NK cells as described in Materials and Methods. Five days after TM_β_1 administration, NK-depleted and isotype-treated mice were injected intravenously with PBS or CD8+ T cells purified from naïve BALB/cJ mice, followed 2 days later by inoculation of EMT6 tumour cells as described in Materials and Methods. S.c. EMT6 tumours were treated with PDT (135 J cm−2 given at 75 mW cm−2) and the average number of lung tumours per mouse was determined as described; error bars represent s.e.m. Open bars represent animals treated with TM_β_1; filled bars represent animals treated with isotype control antibodies. Each group contained a minimum of five mice per group. Significance was calculated by Student's _t_-test. *P<0.0007, when the number of lung tumours per mouse treated with TM_β_1 was compared to the number of lung tumours present per mouse treated with isotype control antibody. #P<0.0001, when the number of lung tumours per mouse with CD8+ T cells and treated with isotype control antibodies was compared to the number of lung tumours per mouse treated with isotype control antibodies. (B) Spleen cells were harvested from CD8+ recipient mice treated with either TM_β_1 or isotype control antibodies at the time of lung tumour assessment. Cells were stained with antibodies specific for CD3, CD8 and CD49; flow cytometry analysis was performed and cells were gated based on CD3 expression. Representative single colour histograms are shown for cells that were CD3+ (left panel) or CD3- (right panel) and expressed either CD8 or CD49 respectively. Filled histograms indicate cells isolated from non-depleted mice stained with isotype control antibodies for anti-CD3 or anti-CD49; open histograms with dashed lines indicate cells isolated from NK depleted mice; open histograms with solid lines indicate cells isolated from non-depleted, isotype treated mice. (C) SCID mice were treated with anti-asialo-GM1 to deplete NK cells as described. Depleted and control mice were inoculated with EMT6 tumours as described in Materials and Methods. S.c. EMT6 tumours were treated with PDT (135 J cm−2 given at 75 mW cm−2) and the average number of lung tumours per mouse was determined as described; error bars represent s.e.m. Open bars represent animals treated with anti-asialo-GM1; filled bars represent animals treated with isotype control antibodies. Each group contained a minimum of five mice per group. Significance was calculated by Student's _t_-test. *P<0.032, when the number of lung tumours per mouse treated with anti-asialo-GM1 was compared to the number of lung tumours present per mouse treated with isotype control antibody. #P<0.0001, when the number of lung tumours per mouse with CD8+ T cells and treated with isotype control antibodies was compared to the number of lung tumours per mouse treated with isotype control antibodies.
Similar articles
- Photodynamic therapy-mediated immune response against subcutaneous mouse tumors.
Korbelik M, Dougherty GJ. Korbelik M, et al. Cancer Res. 1999 Apr 15;59(8):1941-6. Cancer Res. 1999. PMID: 10213504 - Neutralization of interleukin-2 retards the growth of mouse renal cancer.
Fukuhara H, Matsumoto A, Kitamura T, Takeuchi T. Fukuhara H, et al. BJU Int. 2006 Jun;97(6):1314-21. doi: 10.1111/j.1464-410X.2006.06180.x. BJU Int. 2006. PMID: 16686731 - Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease.
Shams M, Owczarczak B, Manderscheid-Kern P, Bellnier DA, Gollnick SO. Shams M, et al. Cancer Immunol Immunother. 2015 Mar;64(3):287-97. doi: 10.1007/s00262-014-1633-9. Epub 2014 Nov 11. Cancer Immunol Immunother. 2015. PMID: 25384911 Free PMC article. - Enhancement of anti-tumor immunity by photodynamic therapy.
Gollnick SO, Brackett CM. Gollnick SO, et al. Immunol Res. 2010 Mar;46(1-3):216-26. doi: 10.1007/s12026-009-8119-4. Immunol Res. 2010. PMID: 19763892 Free PMC article. Review. - Stimulation of anti-tumor immunity by photodynamic therapy.
Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Mroz P, et al. Expert Rev Clin Immunol. 2011 Jan;7(1):75-91. doi: 10.1586/eci.10.81. Expert Rev Clin Immunol. 2011. PMID: 21162652 Free PMC article. Review.
Cited by
- Folate Receptor Targeted Photodynamic Therapy: A Novel Way to Stimulate Anti-Tumor Immune Response in Intraperitoneal Ovarian Cancer.
Baydoun M, Boidin L, Leroux B, Vignion-Dewalle AS, Quilbe A, Grolez GP, Azaïs H, Frochot C, Moralès O, Delhem N. Baydoun M, et al. Int J Mol Sci. 2023 Jul 10;24(14):11288. doi: 10.3390/ijms241411288. Int J Mol Sci. 2023. PMID: 37511049 Free PMC article. - Understanding the Photodynamic Therapy Induced Bystander and Abscopal Effects: A Review.
Moloudi K, Sarbadhikary P, Abrahamse H, George BP. Moloudi K, et al. Antioxidants (Basel). 2023 Jul 16;12(7):1434. doi: 10.3390/antiox12071434. Antioxidants (Basel). 2023. PMID: 37507972 Free PMC article. Review. - Photodynamic priming with triple-receptor targeted nanoconjugates that trigger T cell-mediated immune responses in a 3D in vitro heterocellular model of pancreatic cancer.
De Silva P, Bano S, Pogue BW, Wang KK, Maytin EV, Hasan T. De Silva P, et al. Nanophotonics. 2021 Sep;10(12):3199-3214. doi: 10.1515/nanoph-2021-0304. Epub 2021 Aug 18. Nanophotonics. 2021. PMID: 37485044 Free PMC article. - Trial watch: an update of clinical advances in photodynamic therapy and its immunoadjuvant properties for cancer treatment.
Penetra M, Arnaut LG, Gomes-da-Silva LC. Penetra M, et al. Oncoimmunology. 2023 Jun 18;12(1):2226535. doi: 10.1080/2162402X.2023.2226535. eCollection 2023. Oncoimmunology. 2023. PMID: 37346450 Free PMC article. - Chlorin e6-associated photodynamic therapy enhances abscopal antitumor effects via inhibition of PD-1/PD-L1 immune checkpoint.
Gurung P, Lim J, Shrestha R, Kim YW. Gurung P, et al. Sci Rep. 2023 Mar 21;13(1):4647. doi: 10.1038/s41598-023-30256-0. Sci Rep. 2023. PMID: 36944686 Free PMC article.
References
- Adam C, King S, Allgeier T, Braumuller H, Luking C, Mysliwietz J, Kriegeskorte A, Busch DH, Rocken M, Mocikat R (2005) DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106: 338–344 - PubMed
- Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680 - PubMed
- Bennett SRM, Carbone FR, Karamalis R, Flavell RZ, Miller JFAP, Heath WR (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393: 478–483 - PubMed
- Blank M, Lavie G, Mandel M, Keisari Y (2001) Effects of photodynamic therapy with hypericin in mice bearing highly invasive solid tumors. Oncol Res 12: 409–418 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016056/CA/NCI NIH HHS/United States
- R01 CA098156/CA/NCI NIH HHS/United States
- CA16056/CA/NCI NIH HHS/United States
- P01 CA055791/CA/NCI NIH HHS/United States
- CA55791/CA/NCI NIH HHS/United States
- CA98156/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials