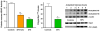Dietary histone deacetylase inhibitors: from cells to mice to man - PubMed (original) (raw)
Review
Dietary histone deacetylase inhibitors: from cells to mice to man
Roderick H Dashwood et al. Semin Cancer Biol. 2007 Oct.
Abstract
Sulforaphane (SFN) is an isothiocyanate found in cruciferous vegetables, such as broccoli and broccoli sprouts. This anticarcinogen was first identified as a potent inducer of Phase 2 detoxification enzymes, but evidence is mounting that SFN also acts through epigenetic mechanisms. SFN has been shown to inhibit histone deacetylase (HDAC) activity in human colon and prostate cancer lines, with an increase in global and local histone acetylation status, such as on the promoter regions of P21 and bax genes. SFN also inhibited the growth of prostate cancer xenografts and spontaneous intestinal polyps in mouse models, with evidence for altered histone acetylation and HDAC activities in vivo. In human subjects, a single ingestion of 68 g broccoli sprouts inhibited HDAC activity in circulating peripheral blood mononuclear cells 3-6 h after consumption, with concomitant induction of histone H3 and H4 acetylation. These findings provide evidence that one mechanism of cancer chemoprevention by SFN is via epigenetic changes associated with inhibition of HDAC activity. Other dietary agents such as butyrate, biotin, lipoic acid, garlic organosulfur compounds, and metabolites of vitamin E have structural features compatible with HDAC inhibition. The ability of dietary compounds to de-repress epigenetically silenced genes in cancer cells, and to activate these genes in normal cells, has important implications for cancer prevention and therapy. In a broader context, there is growing interest in dietary HDAC inhibitors and their impact on epigenetic mechanisms affecting other chronic conditions, such as cardiovascular disease, neurodegeneration and aging.
Figures
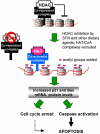
Fig. 1
Working hypothesis for the role of dietary histone deacetylase (HDAC) inhibitors. HDAC/co-repressor complexes maintain a tightly restricted chromatin configuration, which limits access of transcription factors to DNA, and represses genes required for cell cycle checkpoint control and apoptosis. HDAC inhibition by SFN and other dietary agents enables histone acetyltransferase/co-activator (HAT/CoA) complexes to add acetyl groups to histone tails, loosening DNA/chromatin interactions, and allowing access of transcription factors to the promoters of genes such as P21 and bax. Re-expression of these genes facilitates cell cycle arrest and apoptosis in the context of cancer chemoprevention or therapy.
Fig. 2
HDAC inhibition by SFN in human colon and prostate cells, and suppression of xenograft growth in mice. Human colon or prostate cell lines were seeded at ∼1 × 106 and 24 h later treated with SFN. Whole cell lysates or nuclear extracts were obtained after 48 h and examined for protein expression by immunoblotting, or HDAC activity using a commercial kit (BioMol). In chromatin immunoprecipitation (ChIP) assays, antibody to acetylated histone H4 was followed by primers to the promoter region of the P21 gene. In the xenograft studies, 5-week-old male athymic nude BALB/c (nu/nu) mice were randomized to 10 animals per group and fed control AIN93G diet or AIN93G diet containing 443 mg SFN/kg. Human prostate cancer PC-3 cells were mixed in a 1:1 ratio of complete media (RPMI 1640 + 10% FBS) and High Concentration Growth Factors Matrigel Matrix (Becton Dickinson). A suspension of 106 cells (50 μl) was injected subcutaneously into the right flank of each mouse. Tumor volume was calculated using the following formula for the volume of an ellipsoid: length × width2 × 0.5236 (π/6). After 21 days xenografts and host tissues were examined for HDAC activity using the BioMol kit. Error bars indicate mean±S.E.; *P < 0.05, **P < 0.01, ***P < 0.001. Full details of the studies in colon and prostate cells can be found in Refs. [11,17], respectively, and xenograft experiments were reported in Ref. [18]. PBMC, peripheral blood mononuclear cells.
Fig. 3
HDAC inhibition by SFN in mouse colon, and suppression of intestinal polyps. In a short-term pilot study, 3 mice/group were treated by single oral gavage with 10 μmol SFN, 10 μmol of SFN-_N_-acetylcysteine (SFN-NAC), or DMSO vehicle alone. Colonic mucosa was scraped 6 h later and examined in the BioMol HDAC activity assay. In a longer-term experiment, _Apc_min mice ingested on average ∼6 μmol SFN/day for 70 days in AIN93 diet, whereas controls received AIN93 diet alone. Polyps were enumerated at the end of the study, and the intestinal mucosa was immunoblotted for acetylated histones and β-actin. In addition, intestinal mucosa was subjected to ChIP, as described in Fig. 2 legend. Error bars indicate mean±S.E.; **P < 0.01, ***P < 0.001. For further details, see Ref. [19].
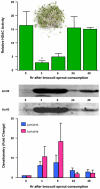
Fig. 4
HDAC inhibition by SFN-rich broccoli sprouts in human volunteers. Three healthy human volunteers refrained from cruciferous vegetable intake for 48 h. Each subject consumed 68 g broccoli sprouts (∼105 mg SFN) with a bagel and cream cheese, and blood was collected at times indicated. PBMCs were immunoblotted for acetylated histones H3 and H4 (acH3, acH4), or the corresponding total histone (H3, H4), and HDAC activities were determined using the BioMol kit. Error bars indicate mean±S.E.; *P < 0.05, _n_=3. For further details, see Ref. [18].
Fig. 5
Dietary HDAC inhibitors. HDAC inhibition has been reported in vitro and/or in vivo for butyrate, garlic organosulfur compounds, and metabolites of SFN, such as SFN-NAC and SFN-Cys, whereas other compounds shown are hypothetical HDAC inhibitors (see text). c9, t11-CLA, cis-9, trans-11-conjugated linoleic acid. Inset: SFN-Cys was manually docked into the active site of human HDAC8, according to the following constraints: (i) carboxylate binding in a bidentate fashion to the active site zinc, (ii) minimal steric conflict between substrate and enzyme, based on a fixed protein, (iii) favorable torsion angles, (iv) hydrogen-bond partners for buried polar atoms, and (v) following the favored position of a bound hydroxamic acid inhibitor [31].
Similar articles
- Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds.
Nian H, Delage B, Ho E, Dashwood RH. Nian H, et al. Environ Mol Mutagen. 2009 Apr;50(3):213-21. doi: 10.1002/em.20454. Environ Mol Mutagen. 2009. PMID: 19197985 Free PMC article. Review. - Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects.
Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Myzak MC, et al. Exp Biol Med (Maywood). 2007 Feb;232(2):227-34. Exp Biol Med (Maywood). 2007. PMID: 17259330 Free PMC article. - A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase.
Myzak MC, Karplus PA, Chung FL, Dashwood RH. Myzak MC, et al. Cancer Res. 2004 Aug 15;64(16):5767-74. doi: 10.1158/0008-5472.CAN-04-1326. Cancer Res. 2004. PMID: 15313918 - Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice.
Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Myzak MC, et al. FASEB J. 2006 Mar;20(3):506-8. doi: 10.1096/fj.05-4785fje. Epub 2006 Jan 11. FASEB J. 2006. PMID: 16407454 Free PMC article. - Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane.
Myzak MC, Dashwood RH. Myzak MC, et al. Curr Drug Targets. 2006 Apr;7(4):443-52. doi: 10.2174/138945006776359467. Curr Drug Targets. 2006. PMID: 16611031 Review.
Cited by
- Vorinostat, an HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth by dampening mTOR signaling pathway in a human xenograft murine model.
Kurundkar D, Srivastava RK, Chaudhary SC, Ballestas ME, Kopelovich L, Elmets CA, Athar M. Kurundkar D, et al. Toxicol Appl Pharmacol. 2013 Jan 15;266(2):233-44. doi: 10.1016/j.taap.2012.11.002. Epub 2012 Nov 9. Toxicol Appl Pharmacol. 2013. PMID: 23147569 Free PMC article. - Safety and tolerability of sauna detoxification for the protracted withdrawal symptoms of substance abuse.
Lennox RD, Cecchini-Sternquist M. Lennox RD, et al. J Int Med Res. 2018 Nov;46(11):4480-4499. doi: 10.1177/0300060518779314. Epub 2018 Sep 13. J Int Med Res. 2018. PMID: 30209965 Free PMC article. - Diet and the epigenetic (re)programming of phenotypic differences in behavior.
McGowan PO, Meaney MJ, Szyf M. McGowan PO, et al. Brain Res. 2008 Oct 27;1237:12-24. doi: 10.1016/j.brainres.2008.07.074. Epub 2008 Jul 29. Brain Res. 2008. PMID: 18694740 Free PMC article. Review. - Impact of dietary gut microbial metabolites on the epigenome.
Gerhauser C. Gerhauser C. Philos Trans R Soc Lond B Biol Sci. 2018 Jun 5;373(1748):20170359. doi: 10.1098/rstb.2017.0359. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29685968 Free PMC article. Review. - Molecular targets of isothiocyanates in cancer: recent advances.
Gupta P, Kim B, Kim SH, Srivastava SK. Gupta P, et al. Mol Nutr Food Res. 2014 Aug;58(8):1685-707. doi: 10.1002/mnfr.201300684. Epub 2014 Feb 10. Mol Nutr Food Res. 2014. PMID: 24510468 Free PMC article. Review.
References
- Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med. 2007;7:85–102. - PubMed
- Fraga MF, Ballestar, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, et al. Loss of acetylation at Lys 16 and trimethylation at Lys 20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. - PubMed
- Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA090890/CA/NCI NIH HHS/United States
- P01 CA090890-01A20003/CA/NCI NIH HHS/United States
- P30 ES00210/ES/NIEHS NIH HHS/United States
- R01 CA080176/CA/NCI NIH HHS/United States
- CA90890/CA/NCI NIH HHS/United States
- R01 CA122906/CA/NCI NIH HHS/United States
- CA80176/CA/NCI NIH HHS/United States
- CA107693/CA/NCI NIH HHS/United States
- R29 CA065525/CA/NCI NIH HHS/United States
- R01 CA065525/CA/NCI NIH HHS/United States
- R01 CA107693/CA/NCI NIH HHS/United States
- CA122906/CA/NCI NIH HHS/United States
- R01 CA065525-09/CA/NCI NIH HHS/United States
- R01 CA122906-01A1/CA/NCI NIH HHS/United States
- R01 CA080176-05/CA/NCI NIH HHS/United States
- CA65525/CA/NCI NIH HHS/United States
- R01 CA065525-10/CA/NCI NIH HHS/United States
- P01 CA090890-01A29001/CA/NCI NIH HHS/United States
- P30 ES000210/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous